Old dogs, new trick: classic cancer therapies activate cGAS
- PMID: 32541866
- PMCID: PMC7395767
- DOI: 10.1038/s41422-020-0346-1
Old dogs, new trick: classic cancer therapies activate cGAS
Abstract
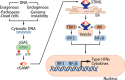
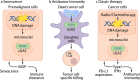
The discovery of cancer immune surveillance and immunotherapy has opened up a new era of cancer treatment. Immunotherapies modulate a patient's immune system to specifically eliminate cancer cells; thus, it is considered a very different approach from classic cancer therapies that usually induce DNA damage to cause cell death in a cell-intrinsic manner. However, recent studies have revealed that classic cancer therapies such as radiotherapy and chemotherapy also elicit antitumor immunity, which plays an essential role in their therapeutic efficacy. The cytosolic DNA sensor cyclic GMP-AMP synthase (cGAS) and the downstream effector Stimulator of Interferon Genes (STING) have been determined to be critical for this interplay. Here, we review the antitumor roles of the cGAS-STING pathway during tumorigenesis, cancer immune surveillance, and cancer therapies. We also highlight classic cancer therapies that elicit antitumor immune responses through cGAS activation.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
cGAS-STING signaling in cancer immunity and immunotherapy.Biomed Pharmacother. 2021 Jan;133:110972. doi: 10.1016/j.biopha.2020.110972. Epub 2020 Nov 27. Biomed Pharmacother. 2021. PMID: 33254021 Review.
-
cGAS/STING cross-talks with cell cycle and potentiates cancer immunotherapy.Mol Ther. 2022 Mar 2;30(3):1006-1017. doi: 10.1016/j.ymthe.2022.01.044. Epub 2022 Feb 2. Mol Ther. 2022. PMID: 35121107 Free PMC article. Review.
-
The Impact of Radiation-Induced DNA Damage on cGAS-STING-Mediated Immune Responses to Cancer.Int J Mol Sci. 2020 Nov 23;21(22):8877. doi: 10.3390/ijms21228877. Int J Mol Sci. 2020. PMID: 33238631 Free PMC article. Review.
-
Cellular senescence and senescence-associated secretory phenotype via the cGAS-STING signaling pathway in cancer.Cancer Sci. 2020 Feb;111(2):304-311. doi: 10.1111/cas.14266. Epub 2019 Dec 27. Cancer Sci. 2020. PMID: 31799772 Free PMC article. Review.
-
Comprehensive elaboration of the cGAS-STING signaling axis in cancer development and immunotherapy.Mol Cancer. 2020 Aug 27;19(1):133. doi: 10.1186/s12943-020-01250-1. Mol Cancer. 2020. PMID: 32854711 Free PMC article. Review.
Cited by
-
Post-Translational Modifications of STING: A Potential Therapeutic Target.Front Immunol. 2022 May 6;13:888147. doi: 10.3389/fimmu.2022.888147. eCollection 2022. Front Immunol. 2022. PMID: 35603197 Free PMC article. Review.
-
Colorectal cancer-specific IFNβ delivery overcomes dysfunctional dsRNA-mediated type I interferon signaling to increase the abscopal effect of radiotherapy.J Immunother Cancer. 2024 May 15;12(5):e008515. doi: 10.1136/jitc-2023-008515. J Immunother Cancer. 2024. PMID: 38749537 Free PMC article.
-
The different biological effects of TMPyP4 and cisplatin in the inflammatory microenvironment of osteosarcoma are attributed to G-quadruplex.Cell Prolif. 2021 Sep;54(9):e13101. doi: 10.1111/cpr.13101. Epub 2021 Jul 23. Cell Prolif. 2021. PMID: 34296479 Free PMC article.
-
PREX2 contributes to radiation resistance by inhibiting radiotherapy-induced tumor immunogenicity via cGAS/STING/IFNs pathway in colorectal cancer.BMC Med. 2024 Apr 12;22(1):154. doi: 10.1186/s12916-024-03375-2. BMC Med. 2024. PMID: 38609982 Free PMC article.
-
Bibliometric and Visualized Analysis of the Current Status on STING Signaling Pathway and Cancer.J Oncol. 2022 Nov 23;2022:5095176. doi: 10.1155/2022/5095176. eCollection 2022. J Oncol. 2022. PMID: 36467504 Free PMC article.
References
-
- Faguet GB. A brief history of cancer: age-old milestones underlying our current knowledge database. Int. J. Cancer. 2015;136:2022–2036. - PubMed
-
- Burnet FM. The concept of immunological surveillance. Prog. Exp. Tumor Res. 1970;13:1–27. - PubMed
-
- Ehrlich P. Ueber den jetzigen stand der Karzinomforschung Nederlandsch Tijdschrift voor Geneeskunde. Ned. Tijdschr. Geneeskd. 1909;5:273–290.
-
- Anichini A, et al. An expanded peripheral T cell population to a cytotoxic T lymphocyte (CTL)-defined, melanocyte-specific antigen in metastatic melanoma patients impacts on generation of peptide-specific CTLs but does not overcome tumor escape from immune surveillance in metastatic lesions. J. Exp. Med. 1999;190:651–667. - PMC - PubMed
-
- Lee PP, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat. Med. 1999;5:677–685. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

