Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability
- PMID: 32540902
- PMCID: PMC7299281
- DOI: 10.1126/science.abc5902
Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability
Abstract
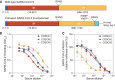
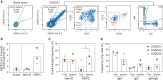
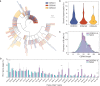
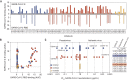
The rapid spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has had a large impact on global health, travel, and economy. Therefore, preventative and therapeutic measures are urgently needed. Here, we isolated monoclonal antibodies from three convalescent coronavirus disease 2019 (COVID-19) patients using a SARS-CoV-2 stabilized prefusion spike protein. These antibodies had low levels of somatic hypermutation and showed a strong enrichment in VH1-69, VH3-30-3, and VH1-24 gene usage. A subset of the antibodies was able to potently inhibit authentic SARS-CoV-2 infection at a concentration as low as 0.007 micrograms per milliliter. Competition and electron microscopy studies illustrate that the SARS-CoV-2 spike protein contains multiple distinct antigenic sites, including several receptor-binding domain (RBD) epitopes as well as non-RBD epitopes. In addition to providing guidance for vaccine design, the antibodies described here are promising candidates for COVID-19 treatment and prevention.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures

Similar articles
-
Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model.Science. 2020 Aug 21;369(6506):956-963. doi: 10.1126/science.abc7520. Epub 2020 Jun 15. Science. 2020. PMID: 32540903 Free PMC article.
-
Potently neutralizing and protective human antibodies against SARS-CoV-2.Nature. 2020 Aug;584(7821):443-449. doi: 10.1038/s41586-020-2548-6. Epub 2020 Jul 15. Nature. 2020. PMID: 32668443 Free PMC article.
-
Structures of Human Antibodies Bound to SARS-CoV-2 Spike Reveal Common Epitopes and Recurrent Features of Antibodies.Cell. 2020 Aug 20;182(4):828-842.e16. doi: 10.1016/j.cell.2020.06.025. Epub 2020 Jun 24. Cell. 2020. PMID: 32645326 Free PMC article.
-
COVID-19 Vaccines: "Warp Speed" Needs Mind Melds, Not Warped Minds.J Virol. 2020 Aug 17;94(17):e01083-20. doi: 10.1128/JVI.01083-20. Print 2020 Aug 17. J Virol. 2020. PMID: 32591466 Free PMC article. Review.
-
Antibodies to SARS-CoV-2 and their potential for therapeutic passive immunization.Elife. 2020 Jun 23;9:e57877. doi: 10.7554/eLife.57877. Elife. 2020. PMID: 32573433 Free PMC article. Review.
Cited by
-
NeutrobodyPlex-monitoring SARS-CoV-2 neutralizing immune responses using nanobodies.EMBO Rep. 2021 May 5;22(5):e52325. doi: 10.15252/embr.202052325. Epub 2021 Apr 27. EMBO Rep. 2021. PMID: 33904225 Free PMC article.
-
Structural insights into the cross-neutralization of SARS-CoV and SARS-CoV-2 by the human monoclonal antibody 47D11.Sci Adv. 2021 Jun 2;7(23):eabf5632. doi: 10.1126/sciadv.abf5632. Print 2021 Jun. Sci Adv. 2021. PMID: 33958322 Free PMC article.
-
Public Baseline and shared response structures support the theory of antibody repertoire functional commonality.PLoS Comput Biol. 2021 Mar 1;17(3):e1008781. doi: 10.1371/journal.pcbi.1008781. eCollection 2021 Mar. PLoS Comput Biol. 2021. PMID: 33647011 Free PMC article.
-
ACE2-based decoy receptors for SARS coronavirus 2.Proteins. 2021 Sep;89(9):1065-1078. doi: 10.1002/prot.26140. Epub 2021 May 18. Proteins. 2021. PMID: 33973262 Free PMC article. Review.
-
A SARS-CoV-2 neutralizing antibody with extensive Spike binding coverage and modified for optimal therapeutic outcomes.Nat Commun. 2021 May 11;12(1):2623. doi: 10.1038/s41467-021-22926-2. Nat Commun. 2021. PMID: 33976198 Free PMC article.
References
-
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L., Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 395, 507–513 (2020). 10.1016/S0140-6736(20)30211-7 - DOI - PMC - PubMed
-
- Mair-Jenkins J., Saavedra-Campos M., Baillie J. K., Cleary P., Khaw F.-M., Lim W. S., Makki S., Rooney K. D., Nguyen-Van-Tam J. S., Beck C. R.; Convalescent Plasma Study Group , The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis. J. Infect. Dis. 211, 80–90 (2015). 10.1093/infdis/jiu396 - DOI - PMC - PubMed
-
- Ko J. H., Seok H., Cho S. Y., Ha Y. E., Baek J. Y., Kim S. H., Kim Y. J., Park J. K., Chung C. R., Kang E.-S., Cho D., Müller M. A., Drosten C., Kang C.-I., Chung D. R., Song J.-H., Peck K. R., Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: A single centre experience. Antivir. Ther. 23, 617–622 (2018). 10.3851/IMP3243 - DOI - PubMed
-
- Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., Wang F., Li D., Yang M., Xing L., Wei J., Xiao H., Yang Y., Qu J., Qing L., Chen L., Xu Z., Peng L., Li Y., Zheng H., Chen F., Huang K., Jiang Y., Liu D., Zhang Z., Liu Y., Liu L., Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 323, 1582 (2020). 10.1001/jama.2020.4783 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

