Nuclear Functions of TOR: Impact on Transcription and the Epigenome
- PMID: 32532005
- PMCID: PMC7349558
- DOI: 10.3390/genes11060641
Nuclear Functions of TOR: Impact on Transcription and the Epigenome
Abstract
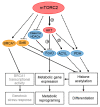
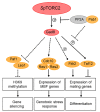
The target of rapamycin (TOR) protein kinase is at the core of growth factor- and nutrient-dependent signaling pathways that are well-known for their regulation of metabolism, growth, and proliferation. However, TOR is also involved in the regulation of gene expression, genomic and epigenomic stability. TOR affects nuclear functions indirectly through its activity in the cytoplasm, but also directly through active nuclear TOR pools. The mechanisms by which TOR regulates its nuclear functions are less well-understood compared with its cytoplasmic activities. TOR is an important pharmacological target for several diseases, including cancer, metabolic and neurological disorders. Thus, studies of the nuclear functions of TOR are important for our understanding of basic biological processes, as well as for clinical implications.
Keywords: TORC1; TORC2; acetylation; cancer; epigenetics; genomic stability; histones; methylation; target of rapamycin; transcription.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the analyses or interpretation of data, in the writing of the manuscript, or in the decision to publish results.
Figures

Similar articles
-
Environmental signaling through the mechanistic target of rapamycin complex 1: mTORC1 goes nuclear.Cell Cycle. 2014;13(5):714-25. doi: 10.4161/cc.28112. Epub 2014 Feb 7. Cell Cycle. 2014. PMID: 24526113 Free PMC article. Review.
-
Gad8 Protein Is Found in the Nucleus Where It Interacts with the MluI Cell Cycle Box-binding Factor (MBF) Transcriptional Complex to Regulate the Response to DNA Replication Stress.J Biol Chem. 2016 Apr 22;291(17):9371-81. doi: 10.1074/jbc.M115.705251. Epub 2016 Feb 24. J Biol Chem. 2016. PMID: 26912660 Free PMC article.
-
Evolutionarily conserved regulation of TOR signalling.J Biochem. 2013 Jul;154(1):1-10. doi: 10.1093/jb/mvt047. Epub 2013 May 21. J Biochem. 2013. PMID: 23698095 Review.
-
Target of Rapamycin Complex 2 regulates cell growth via Myc in Drosophila.Sci Rep. 2015 May 22;5:10339. doi: 10.1038/srep10339. Sci Rep. 2015. PMID: 25999153 Free PMC article.
-
Target of Rapamycin (TOR) Regulates Growth in Response to Nutritional Signals.Microbiol Spectr. 2016 Oct;4(5). doi: 10.1128/microbiolspec.FUNK-0006-2016. Microbiol Spectr. 2016. PMID: 27763256 Review.
Cited by
-
Beta-amyloid Deposition in Biliary Atresia Reduces Liver Regeneration by Inhibiting Energy Metabolism and Mammalian Target of Rapamycin Signaling.Clin Transl Gastroenterol. 2022 Nov 1;13(11):e00536. doi: 10.14309/ctg.0000000000000536. Clin Transl Gastroenterol. 2022. PMID: 36137184 Free PMC article.
-
Inorganic polyphosphate abets silencing of a sub-telomeric gene cluster in fission yeast.MicroPubl Biol. 2023 Feb 3;2023:10.17912/micropub.biology.000744. doi: 10.17912/micropub.biology.000744. eCollection 2023. MicroPubl Biol. 2023. PMID: 36820394 Free PMC article.
-
mTORC2 interactome and localization determine aggressiveness of high-grade glioma cells through association with gelsolin.Sci Rep. 2023 Apr 29;13(1):7037. doi: 10.1038/s41598-023-33872-y. Sci Rep. 2023. PMID: 37120454 Free PMC article.
-
Hierarchical Phosphorylation of HOXB13 by mTOR Dictates Its Activity and Oncogenic Function in Prostate Cancer.Mol Cancer Res. 2023 Oct 2;21(10):1050-1063. doi: 10.1158/1541-7786.MCR-23-0086. Mol Cancer Res. 2023. PMID: 37409967 Free PMC article.
-
TOR Complex 2- independent mutations in the regulatory PIF pocket of Gad8AKT1/SGK1 define separate branches of the stress response mechanisms in fission yeast.PLoS Genet. 2020 Nov 2;16(11):e1009196. doi: 10.1371/journal.pgen.1009196. eCollection 2020 Nov. PLoS Genet. 2020. PMID: 33137119 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

