Negative control of diacylglycerol kinase ζ-mediated inhibition of T cell receptor signaling by nuclear sequestration in mice
- PMID: 32525220
- PMCID: PMC7705894
- DOI: 10.1002/eji.201948442
Negative control of diacylglycerol kinase ζ-mediated inhibition of T cell receptor signaling by nuclear sequestration in mice
Abstract
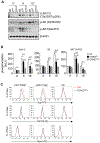
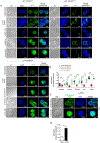
Diacylglycerol kinases (DGKs) play important roles in restraining diacylglycerol (DAG)-mediated signaling. Within the DGK family, the ζ isoform appears to be the most important isoform in T cells for controlling their development and function. DGKζ has been demonstrated to regulate T cell maturation, activation, anergy, effector/memory differentiation, defense against microbial infection, and antitumor immunity. Given its critical functions, DGKζ function should be tightly regulated to ensure proper signal transduction; however, mechanisms that control DGKζ function are still poorly understood. We report here that DGKζ dynamically translocates from the cytosol into the nuclei in T cells after TCR stimulation. In mice, DGKζ mutant defective in nuclear localization displayed enhanced ability to inhibit TCR-induced DAG-mediated signaling in primary T cells, maturation of conventional αβT and iNKT cells, and activation of peripheral T cells compared with WT DGKζ. Our study reveals for the first time nuclear sequestration of DGKζ as a negative control mechanism to spatially restrain it from terminating DAG mediated signaling in T cells. Our data suggest that manipulation of DGKζ nucleus-cytosol shuttling as a novel strategy to modulate DGKζ activity and immune responses for treatment of autoimmune diseases and cancer.
Keywords: Ras/MAPK; T cell development; TCR; diacylglycerol kinases; invariant NKT cell.
© 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Conflict of Interest Disclosures
The authors declare no commercial or financial conflict of interests.
Figures





Similar articles
-
The ζ isoform of diacylglycerol kinase plays a predominant role in regulatory T cell development and TCR-mediated ras signaling.Sci Signal. 2013 Nov 26;6(303):ra102. doi: 10.1126/scisignal.2004373. Sci Signal. 2013. PMID: 24280043 Free PMC article.
-
Diacylglycerol kinase ζ controls diacylglycerol metabolism at the immunological synapse.Mol Biol Cell. 2011 Nov;22(22):4406-14. doi: 10.1091/mbc.E11-03-0247. Epub 2011 Sep 21. Mol Biol Cell. 2011. PMID: 21937721 Free PMC article.
-
Manipulation of diacylglycerol and ERK-mediated signaling differentially controls CD8+ T cell responses during chronic viral infection.Front Immunol. 2022 Nov 24;13:1032113. doi: 10.3389/fimmu.2022.1032113. eCollection 2022. Front Immunol. 2022. PMID: 36846018 Free PMC article.
-
Redundant and specialized roles for diacylglycerol kinases α and ζ in the control of T cell functions.Sci Signal. 2015 Apr 28;8(374):re6. doi: 10.1126/scisignal.aaa0974. Sci Signal. 2015. PMID: 25921290 Review.
-
Diacylglycerol kinases in the regulation of dendritic spines.J Neurochem. 2010 Feb;112(3):577-87. doi: 10.1111/j.1471-4159.2009.06499.x. Epub 2009 Nov 17. J Neurochem. 2010. PMID: 19922438 Review.
Cited by
-
Differential controls of MAIT cell effector polarization by mTORC1/mTORC2 via integrating cytokine and costimulatory signals.Nat Commun. 2021 Apr 1;12(1):2029. doi: 10.1038/s41467-021-22162-8. Nat Commun. 2021. PMID: 33795689 Free PMC article.
References
-
- Merida I, Arranz-Nicolas J, Rodriguez-Rodriguez C and Avila-Flores A, Diacylglycerol kinase control of protein kinase C. Biochem J 2019. 476: 1205–1219. - PubMed
-
- Mariathasan S, Zakarian A, Bouchard D, Michie AM, Zuniga-Pflucker JC and Ohashi PS, Duration and strength of extracellular signal-regulated kinase signals are altered during positive versus negative thymocyte selection. J Immunol 2001. 167: 4966–4973. - PubMed
-
- Alberola-Ila J and Hernandez-Hoyos G, The Ras/MAPK cascade and the control of positive selection. Immunol Rev 2003. 191: 79–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

