Pharmacological activity and NMR solution structure of the leech peptide HSTX-I
- PMID: 32524995
- PMCID: PMC8494138
- DOI: 10.1016/j.bcp.2020.114082
Pharmacological activity and NMR solution structure of the leech peptide HSTX-I
Abstract
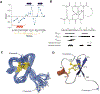
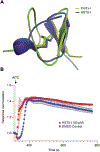
The role of voltage-gated sodium (NaV) channels in pain perception is indisputable. Of particular interest as targets for the development of pain therapeutics are the tetrodotoxin-resistant isoforms NaV1.8 and NaV1.9, based on animal as well as human genetic studies linking these ion channel subtypes to the pathogenesis of pain. However, only a limited number of inhibitors selectively targeting these channels have been reported. HSTX-I is a peptide toxin identified from saliva of the leech Haemadipsa sylvestris. The native 23-residue peptide, stabilised by two disulfide bonds, has been reported to inhibit rat NaV1.8 and mouse NaV1.9 with low micromolar activity, and may therefore represent a scaffold for development of novel modulators with activity at human tetrodotoxin-resistant NaV isoforms. We synthetically produced this hydrophobic peptide in high yield using a one-pot oxidation and single step purification and determined the three-dimensional solution structure of HSTX-I using NMR solution spectroscopy. However, in our hands, the synthetic HSTX-I displayed only very modest activity at human NaV1.8 and NaV1.9, and lacked analgesic efficacy in a murine model of inflammatory pain.
Keywords: Chronic pain; Electrophysiology; Leech; NMR; Voltage-gated sodium channels.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


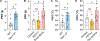
Similar articles
-
Pharmacological characterization of potent and selective NaV1.7 inhibitors engineered from Chilobrachys jingzhao tarantula venom peptide JzTx-V.PLoS One. 2018 May 3;13(5):e0196791. doi: 10.1371/journal.pone.0196791. eCollection 2018. PLoS One. 2018. PMID: 29723257 Free PMC article.
-
Mutational analysis of ProTx-I and the novel venom peptide Pe1b provide insight into residues responsible for selective inhibition of the analgesic drug target NaV1.7.Biochem Pharmacol. 2020 Nov;181:114080. doi: 10.1016/j.bcp.2020.114080. Epub 2020 Jun 6. Biochem Pharmacol. 2020. PMID: 32511987
-
Voltage-gated sodium channel function and expression in injured and uninjured rat dorsal root ganglia neurons.Int J Neurosci. 2016;126(2):182-92. doi: 10.3109/00207454.2015.1004172. Epub 2015 Apr 7. Int J Neurosci. 2016. PMID: 25562420
-
Selective Voltage-Gated Sodium Channel Peptide Toxins from Animal Venom: Pharmacological Probes and Analgesic Drug Development.ACS Chem Neurosci. 2018 Feb 21;9(2):187-197. doi: 10.1021/acschemneuro.7b00406. Epub 2017 Dec 8. ACS Chem Neurosci. 2018. PMID: 29161016 Review.
-
Scorpion Toxins Targeting Voltage-gated Sodium Channels Associated with Pain.Curr Pharm Biotechnol. 2018;19(11):848-855. doi: 10.2174/1389201019666181105160744. Curr Pharm Biotechnol. 2018. PMID: 30398114 Review.
Cited by
-
The influence of Nav1.9 channels on intestinal hyperpathia and dysmotility.Channels (Austin). 2023 Dec;17(1):2212350. doi: 10.1080/19336950.2023.2212350. Channels (Austin). 2023. PMID: 37186898 Free PMC article. Review.
-
Isolation and Characterization of Poeciguamerin, a Peptide with Dual Analgesic and Anti-Thrombotic Activity from the Poecilobdella manillensis Leech.Int J Mol Sci. 2023 Jul 4;24(13):11097. doi: 10.3390/ijms241311097. Int J Mol Sci. 2023. PMID: 37446275 Free PMC article.
References
-
- Hille B (Ed.), Ion Channels of Excitable Membrane, third ed., Sinauer Associates INC., Massachusetts, 2001.
-
- Vetter I, Deuis JR, Mueller A, Israel MR, Starobova H, Zhang A, et al., NaV1.7 as a pain target - From gene to pharmacology, Pharmacol. Ther. 172 (2017) 73–100. - PubMed
-
- Cao L, McDonnell A, Nitzsche A, Alexandrou A, Saintot PP, Loucif AJ, et al., Pharmacological reversal of a pain phenotype in iPSC-derived sensory neurons and patients with inherited erythromelalgia, Sci. Transl. Med. 8 (2016) 335–356. - PubMed
-
- Bennett DL, Clark AJ, Huang J, Waxman SG, Dib-Hajj SD, The role of voltage-gated sodium channels in pain signaling, Physiol. Rev. 99 (2019) 1079–1151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

