Phospho-Ser784-VCP Is Required for DNA Damage Response and Is Associated with Poor Prognosis of Chemotherapy-Treated Breast Cancer
- PMID: 32521270
- PMCID: PMC7282751
- DOI: 10.1016/j.celrep.2020.107745
Phospho-Ser784-VCP Is Required for DNA Damage Response and Is Associated with Poor Prognosis of Chemotherapy-Treated Breast Cancer
Abstract
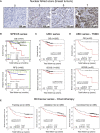
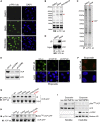
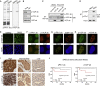
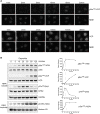
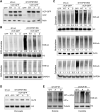
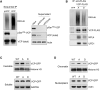
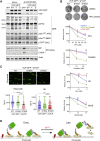
Spatiotemporal protein reorganization at DNA damage sites induced by genotoxic chemotherapies is crucial for DNA damage response (DDR), which influences treatment response by directing cancer cell fate. This process is orchestrated by valosin-containing protein (VCP), an AAA+ ATPase that extracts polyubiquinated chromatin proteins and facilitates their turnover. However, because of the essential and pleiotropic effects of VCP in global proteostasis, it remains challenging practically to understand and target its DDR-specific functions. We describe a DNA-damage-induced phosphorylation event (Ser784), which selectively enhances chromatin-associated protein degradation mediated by VCP and is required for DNA repair, signaling, and cell survival. These functional effects of Ser784 phosphorylation on DDR correlate with a decrease in VCP association with chromatin, cofactors NPL4/UFD1, and polyubiquitinated substrates. Clinically, high phospho-Ser784-VCP levels are significantly associated with poor outcome among chemotherapy-treated breast cancer patients. Thus, Ser784 phosphorylation is a DDR-specific enhancer of VCP function and a potential predictive biomarker for chemotherapy treatments.
Keywords: DNA damage response; K48-linked polyubiquitin; VCP; biomarker; cancer; chemotherapy; chromatin-associated degradation; nucleus; phosphorylation; proteostasis.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests A provisional patent has been filed by J.S. for the monoclonal pSer(784)-VCP antibody for its potential value as a prognostic and predictive cancer biomarker. T.O.N. had a role in the development of the PAM50 gene-expression classifier, which has been licensed to Veracyte Technologies.
Figures
Similar articles
-
Phospho-Ser784-VCP Drives Resistance of Pancreatic Ductal Adenocarcinoma to Genotoxic Chemotherapies and Predicts the Chemo-Sensitizing Effect of VCP Inhibitor.Cancers (Basel). 2021 Oct 11;13(20):5076. doi: 10.3390/cancers13205076. Cancers (Basel). 2021. PMID: 34680224 Free PMC article.
-
Ser784 phosphorylation: a clinically relevant enhancer of VCP function in the DNA damage response.Mol Cell Oncol. 2020 Aug 12;7(5):1796179. doi: 10.1080/23723556.2020.1796179. eCollection 2020. Mol Cell Oncol. 2020. PMID: 32944647 Free PMC article.
-
Valosin-containing Protein (VCP)/p97 Segregase Mediates Proteolytic Processing of Cockayne Syndrome Group B (CSB) in Damaged Chromatin.J Biol Chem. 2016 Apr 1;291(14):7396-408. doi: 10.1074/jbc.M115.705350. Epub 2016 Jan 29. J Biol Chem. 2016. PMID: 26826127 Free PMC article.
-
Identification of an AGO (Argonaute) protein as a prey of TER94/VCP.Autophagy. 2020 Jan;16(1):190-192. doi: 10.1080/15548627.2019.1691351. Epub 2019 Nov 12. Autophagy. 2020. PMID: 31718417 Free PMC article. Review.
-
Expanding into new markets--VCP/p97 in endocytosis and autophagy.J Struct Biol. 2012 Aug;179(2):78-82. doi: 10.1016/j.jsb.2012.03.003. Epub 2012 Mar 19. J Struct Biol. 2012. PMID: 22450227 Review.
Cited by
-
Phospho-Ser784-VCP Drives Resistance of Pancreatic Ductal Adenocarcinoma to Genotoxic Chemotherapies and Predicts the Chemo-Sensitizing Effect of VCP Inhibitor.Cancers (Basel). 2021 Oct 11;13(20):5076. doi: 10.3390/cancers13205076. Cancers (Basel). 2021. PMID: 34680224 Free PMC article.
-
Valosin-Containing Protein (VCP)/p97: A Prognostic Biomarker and Therapeutic Target in Cancer.Int J Mol Sci. 2021 Sep 21;22(18):10177. doi: 10.3390/ijms221810177. Int J Mol Sci. 2021. PMID: 34576340 Free PMC article. Review.
-
The protein segregase VCP/p97 promotes host antifungal defense via regulation of SYK activation.PLoS Pathog. 2024 Oct 29;20(10):e1012674. doi: 10.1371/journal.ppat.1012674. eCollection 2024 Oct. PLoS Pathog. 2024. PMID: 39471181 Free PMC article.
-
Ser784 phosphorylation: a clinically relevant enhancer of VCP function in the DNA damage response.Mol Cell Oncol. 2020 Aug 12;7(5):1796179. doi: 10.1080/23723556.2020.1796179. eCollection 2020. Mol Cell Oncol. 2020. PMID: 32944647 Free PMC article.
-
Inhibition of VCP modulates NF-κB signaling pathway to suppress multiple myeloma cell proliferation and osteoclast differentiation.Aging (Albany NY). 2023 Aug 21;15(16):8220-8236. doi: 10.18632/aging.204965. Epub 2023 Aug 21. Aging (Albany NY). 2023. PMID: 37606987 Free PMC article.
References
-
- Acs K., Luijsterburg M.S., Ackermann L., Salomons F.A., Hoppe T., Dantuma N.P. The AAA-ATPase VCP/p97 promotes 53BP1 recruitment by removing L3MBTL1 from DNA double-strand breaks. Nat. Struct. Mol. Biol. 2011;18:1345–1350. - PubMed
-
- Awasthi P., Foiani M., Kumar A. ATM and ATR signaling at a glance. J. Cell Sci. 2016;129:1285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

