Epithelial tissue geometry directs emergence of bioelectric field and pattern of proliferation
- PMID: 32520653
- PMCID: PMC7521849
- DOI: 10.1091/mbc.E19-12-0719
Epithelial tissue geometry directs emergence of bioelectric field and pattern of proliferation
Abstract
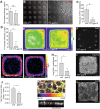
Patterns of proliferation are templated by both gradients of mechanical stress as well as by gradients in membrane voltage (Vm), which is defined as the electric potential difference between the cytoplasm and the extracellular medium. Either gradient could regulate the emergence of the other, or they could arise independently and synergistically affect proliferation within a tissue. Here, we examined the relationship between endogenous patterns of mechanical stress and the generation of bioelectric gradients in mammary epithelial tissues. We observed that the mechanical stress gradients in the tissues presaged gradients in both proliferation and depolarization, consistent with previous reports correlating depolarization with proliferation. Furthermore, disrupting the Vm gradient blocked the emergence of patterned proliferation. We found that the bioelectric gradient formed downstream of mechanical stresses within the tissues and depended on connexin-43 (Cx43) hemichannels, which opened preferentially in cells located in regions of high mechanical stress. Activation of Cx43 hemichannels was necessary for nuclear localization of Yap/Taz and induction of proliferation. Together, these results suggest that mechanotransduction triggers the formation of bioelectric gradients across a tissue, which are further translated into transcriptional changes that template patterns of growth.
Figures
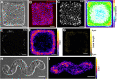
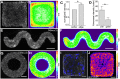
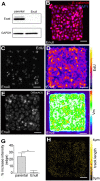

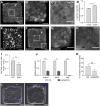

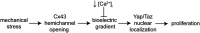
Comment in
-
Editorial introduction.Mol Biol Cell. 2020 Jul 21;31(16):1651-1653. doi: 10.1091/mbc.E20-06-0414. Mol Biol Cell. 2020. PMID: 32692641 Free PMC article.
Similar articles
-
Osteocytic connexin hemichannels suppress breast cancer growth and bone metastasis.Oncogene. 2016 Oct 27;35(43):5597-5607. doi: 10.1038/onc.2016.101. Epub 2016 Apr 4. Oncogene. 2016. PMID: 27041582 Free PMC article.
-
Endogenous patterns of mechanical stress are required for branching morphogenesis.Integr Biol (Camb). 2010 Sep;2(9):424-34. doi: 10.1039/c0ib00040j. Epub 2010 Aug 17. Integr Biol (Camb). 2010. PMID: 20717570 Free PMC article.
-
The Hippo pathway integrates PI3K-Akt signals with mechanical and polarity cues to control tissue growth.PLoS Biol. 2019 Oct 15;17(10):e3000509. doi: 10.1371/journal.pbio.3000509. eCollection 2019 Oct. PLoS Biol. 2019. PMID: 31613895 Free PMC article.
-
YAP and TAZ in epithelial stem cells: A sensor for cell polarity, mechanical forces and tissue damage.Bioessays. 2016 Jul;38(7):644-53. doi: 10.1002/bies.201600037. Epub 2016 May 13. Bioessays. 2016. PMID: 27173018 Free PMC article. Review.
-
Connexin targeting peptides as inhibitors of voltage- and intracellular Ca2+-triggered Cx43 hemichannel opening.Neuropharmacology. 2013 Dec;75:506-16. doi: 10.1016/j.neuropharm.2013.08.021. Epub 2013 Sep 2. Neuropharmacology. 2013. PMID: 24007825 Review.
Cited by
-
Optical Control of Tissue Regeneration through Photostimulation of Organic Semiconducting Nanoparticles.Adv Healthc Mater. 2022 Oct;11(19):e2200366. doi: 10.1002/adhm.202200366. Epub 2022 Jul 28. Adv Healthc Mater. 2022. PMID: 35861262 Free PMC article.
-
Substratum stiffness tunes membrane voltage in mammary epithelial cells.J Cell Sci. 2021 Jul 1;134(13):jcs256313. doi: 10.1242/jcs.256313. Epub 2021 Jul 12. J Cell Sci. 2021. PMID: 34313313 Free PMC article.
-
Information integration during bioelectric regulation of morphogenesis of the embryonic frog brain.iScience. 2023 Nov 4;26(12):108398. doi: 10.1016/j.isci.2023.108398. eCollection 2023 Dec 15. iScience. 2023. PMID: 38034358 Free PMC article.
-
In situ modulation of intestinal organoid epithelial curvature through photoinduced viscoelasticity directs crypt morphogenesis.Sci Adv. 2023 Jan 20;9(3):eadd5668. doi: 10.1126/sciadv.add5668. Epub 2023 Jan 20. Sci Adv. 2023. PMID: 36662859 Free PMC article.
-
Engineering tools for quantifying and manipulating forces in epithelia.Biophys Rev (Melville). 2023 May 11;4(2):021303. doi: 10.1063/5.0142537. eCollection 2023 Jun. Biophys Rev (Melville). 2023. PMID: 38510344 Free PMC article. Review.
References
-
- Adams D.S., Masi A., Levin M. (2007). H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development , 1323–1335. - PubMed
-
- Alenghat F.J., Nauli S.M., Kolb R., Zhou J., Ingber D.E. (2004). Global cytoskeletal control of mechanotransduction in kidney epithelial cells. Exp Cell Res , 23–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

