An IFT20 mechanotrafficking axis is required for integrin recycling, focal adhesion dynamics, and polarized cell migration
- PMID: 32520638
- PMCID: PMC7525813
- DOI: 10.1091/mbc.E20-04-0232
An IFT20 mechanotrafficking axis is required for integrin recycling, focal adhesion dynamics, and polarized cell migration
Abstract
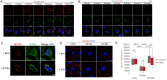
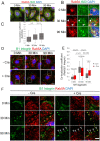
Directional cell migration drives embryonic development, cancer metastasis, and tissue repair and regeneration. Here, we examine the role of intraflagellar transport (IFT) 20 (Ift20) during polarized migration of epidermal cells. IFT20 is implicated in regulating cell migration independently of the primary cilium, but how IFT proteins integrate with the cell migration machinery is poorly understood. We show that genetic ablation of IFT20 in vitro slows keratinocyte migration during wound healing. We find that this phenotype is independent of the primary cilium and instead can be attributed to alterations in integrin-mediated mechanotransduction and focal adhesion (FA) dynamics. Loss of Ift20 resulted in smaller and less numerous FAs and reduced the levels of activated FA kinase. Studies of FA dynamics during microtubule-induced FA turnover demonstrated that Ift20 loss specifically impaired the reformation, but not the disassembly, of FAs. In the absence of Ift20 function, β1 integrins endocytosed during FA disassembly are not transferred out of Rab5 (+) endosomes. This defective transit from the early endosome disrupts eventual recycling of β1 integrins back to the cell surface, resulting in defective FA reformation. In vivo, conditional ablation of Ift20 in hair follicle stem cells (HF-SCs) similarly impairs their ability to invade and migrate during epidermal wound healing. Using explant studies, lineage tracing, and clonal analysis, we demonstrate that Ift20 is required for HF-SC migration and their contribution to epidermal regeneration. This work identifies a new Ift20 mechanotrafficking mechanism required for polarized cell migration and stem cell-driven tissue repair.
Figures

Similar articles
-
FAK, talin and PIPKIγ regulate endocytosed integrin activation to polarize focal adhesion assembly.Nat Cell Biol. 2016 May;18(5):491-503. doi: 10.1038/ncb3333. Epub 2016 Apr 4. Nat Cell Biol. 2016. PMID: 27043085
-
Kindlin-1 regulates integrin dynamics and adhesion turnover.PLoS One. 2013 Jun 11;8(6):e65341. doi: 10.1371/journal.pone.0065341. Print 2013. PLoS One. 2013. PMID: 23776470 Free PMC article.
-
EHD1 regulates beta1 integrin endosomal transport: effects on focal adhesions, cell spreading and migration.J Cell Sci. 2007 Mar 1;120(Pt 5):802-14. doi: 10.1242/jcs.03383. Epub 2007 Feb 6. J Cell Sci. 2007. PMID: 17284518
-
Mechanotransduction in endothelial cell migration.J Cell Biochem. 2005 Dec 15;96(6):1110-26. doi: 10.1002/jcb.20614. J Cell Biochem. 2005. PMID: 16167340 Review.
-
Microtubules in cell migration.Essays Biochem. 2019 Oct 31;63(5):509-520. doi: 10.1042/EBC20190016. Essays Biochem. 2019. PMID: 31358621 Free PMC article. Review.
Cited by
-
The ARF GAPs ELMOD1 and ELMOD3 act at the Golgi and cilia to regulate ciliogenesis and ciliary protein traffic.Mol Biol Cell. 2022 Feb 1;33(2):ar13. doi: 10.1091/mbc.E21-09-0443. Epub 2021 Nov 24. Mol Biol Cell. 2022. PMID: 34818063 Free PMC article.
-
Feasibility of repairing skin defects by VEGF165 gene-modified iPS-HFSCs seeded on a 3D printed scaffold containing astragalus polysaccharide.J Cell Mol Med. 2023 Aug;27(15):2136-2149. doi: 10.1111/jcmm.17800. Epub 2023 Jun 1. J Cell Mol Med. 2023. PMID: 37264501 Free PMC article.
-
IFT20 Mediates the Transport of Cell Migration Regulators From the Trans-Golgi Network to the Plasma Membrane in Breast Cancer Cells.Front Cell Dev Biol. 2021 Feb 26;9:632198. doi: 10.3389/fcell.2021.632198. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33748116 Free PMC article.
-
IFT20: An Eclectic Regulator of Cellular Processes beyond Intraflagellar Transport.Int J Mol Sci. 2022 Oct 12;23(20):12147. doi: 10.3390/ijms232012147. Int J Mol Sci. 2022. PMID: 36292997 Free PMC article. Review.
References
-
- Behne MJ, Tu CL, Aronchik I, Epstein E, Bench G, Bikle DD, Pozzan T, Mauro TM. (2003). Human keratinocyte ATP2C1 localizes to the Golgi and controls Golgi Ca2+ stores. J Invest Dermatol , 688–694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

