Talin-1 is the principal platelet Rap1 effector of integrin activation
- PMID: 32518959
- PMCID: PMC7472713
- DOI: 10.1182/blood.2020005348
Talin-1 is the principal platelet Rap1 effector of integrin activation
Abstract
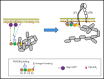
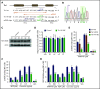
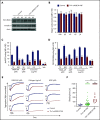
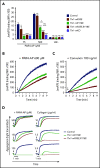
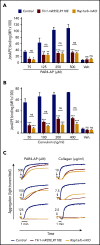
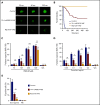
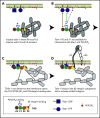
Ras-related protein 1 (Rap1) is a major convergence point of the platelet-signaling pathways that result in talin-1 binding to the integrin β cytoplasmic domain and consequent integrin activation, platelet aggregation, and effective hemostasis. The nature of the connection between Rap1 and talin-1 in integrin activation is an important remaining gap in our understanding of this process. Previous work identified a low-affinity Rap1-binding site in the talin-1 F0 domain that makes a small contribution to integrin activation in platelets. We recently identified an additional Rap1-binding site in the talin-1 F1 domain that makes a greater contribution than F0 in model systems. Here we generated mice bearing point mutations, which block Rap1 binding without affecting talin-1 expression, in either the talin-1 F1 domain (R118E) alone, which were viable, or in both the F0 and F1 domains (R35E,R118E), which were embryonic lethal. Loss of the Rap1-talin-1 F1 interaction in platelets markedly decreases talin-1-mediated activation of platelet β1- and β3-integrins. Integrin activation and platelet aggregation in mice whose platelets express only talin-1(R35E, R118E) are even more impaired, resembling the defect seen in platelets lacking both Rap1a and Rap1b. Although Rap1 is important in thrombopoiesis, platelet secretion, and surface exposure of phosphatidylserine, loss of the Rap1-talin-1 interaction in talin-1(R35E, R118E) platelets had little effect on these processes. These findings show that talin-1 is the principal direct effector of Rap1 GTPases that regulates platelet integrin activation in hemostasis.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
Similar articles
-
Rap1 binding to the talin 1 F0 domain makes a minimal contribution to murine platelet GPIIb-IIIa activation.Blood Adv. 2018 Sep 25;2(18):2358-2368. doi: 10.1182/bloodadvances.2018020487. Blood Adv. 2018. PMID: 30242097 Free PMC article.
-
Functional redundancy between RAP1 isoforms in murine platelet production and function.Blood. 2018 Nov 1;132(18):1951-1962. doi: 10.1182/blood-2018-03-838714. Epub 2018 Aug 21. Blood. 2018. PMID: 30131434 Free PMC article.
-
The N-terminal domains of talin cooperate with the phosphotyrosine binding-like domain to activate beta1 and beta3 integrins.J Biol Chem. 2008 Mar 7;283(10):6118-25. doi: 10.1074/jbc.M709527200. Epub 2007 Dec 28. J Biol Chem. 2008. PMID: 18165225
-
Integrin activation.Biochem Soc Trans. 2008 Apr;36(Pt 2):229-34. doi: 10.1042/BST0360229. Biochem Soc Trans. 2008. PMID: 18363565 Free PMC article. Review.
-
RAP GTPases and platelet integrin signaling.Platelets. 2019;30(1):41-47. doi: 10.1080/09537104.2018.1476681. Epub 2018 Jun 4. Platelets. 2019. PMID: 29863951 Free PMC article. Review.
Cited by
-
Mechanism of integrin activation by talin and its cooperation with kindlin.Nat Commun. 2022 Apr 29;13(1):2362. doi: 10.1038/s41467-022-30117-w. Nat Commun. 2022. PMID: 35488005 Free PMC article.
-
Endothelial cells regulate alveolar morphogenesis by constructing basement membranes acting as a scaffold for myofibroblasts.Nat Commun. 2024 Mar 4;15(1):1622. doi: 10.1038/s41467-024-45910-y. Nat Commun. 2024. PMID: 38438343 Free PMC article.
-
Network pharmacology and in vivo evidence of the pharmacological mechanism of geniposide in the treatment of atherosclerosis.BMC Complement Med Ther. 2024 Jan 24;24(1):53. doi: 10.1186/s12906-024-04356-x. BMC Complement Med Ther. 2024. PMID: 38267978 Free PMC article.
-
Molecular mechanisms of leukocyte β2 integrin activation.Blood. 2022 Jun 16;139(24):3480-3492. doi: 10.1182/blood.2021013500. Blood. 2022. PMID: 35167661 Free PMC article. Review.
-
Genes involved in platelet aggregation and activation are downregulated during acute anaphylaxis in humans.Clin Transl Immunology. 2022 Dec 26;11(12):e1435. doi: 10.1002/cti2.1435. eCollection 2022. Clin Transl Immunology. 2022. PMID: 36583159 Free PMC article.
References
-
- McEver RP, Bennett EM, Martin MN. Identification of two structurally and functionally distinct sites on human platelet membrane glycoprotein IIb-IIIa using monoclonal antibodies. J Biol Chem. 1983;258(8):5269-5275. - PubMed
-
- Ginsberg M, Pierschbacher MD, Ruoslahti E, Marguerie G, Plow E. Inhibition of fibronectin binding to platelets by proteolytic fragments and synthetic peptides which support fibroblast adhesion. J Biol Chem. 1985;260(7):3931-3936. - PubMed
-
- Kloczewiak M, Timmons S, Lukas TJ, Hawiger J. Platelet receptor recognition site on human fibrinogen. Synthesis and structure-function relationship of peptides corresponding to the carboxy-terminal segment of the gamma chain. Biochemistry. 1984;23(8):1767-1774. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

