The Intriguing Effects of Substituents in the N-Phenethyl Moiety of Norhydromorphone: A Bifunctional Opioid from a Set of "Tail Wags Dog" Experiments
- PMID: 32517185
- PMCID: PMC7321161
- DOI: 10.3390/molecules25112640
The Intriguing Effects of Substituents in the N-Phenethyl Moiety of Norhydromorphone: A Bifunctional Opioid from a Set of "Tail Wags Dog" Experiments
Abstract
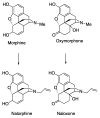
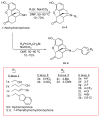
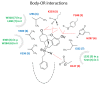
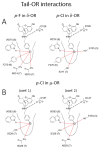
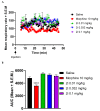
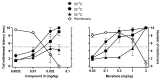
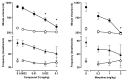
(-)-N-Phenethyl analogs of optically pure N-norhydromorphone were synthesized and pharmacologically evaluated in several in vitro assays (opioid receptor binding, stimulation of [35S]GTPγS binding, forskolin-induced cAMP accumulation assay, and MOR-mediated β-arrestin recruitment assays). "Body" and "tail" interactions with opioid receptors (a subset of Portoghese's message-address theory) were used for molecular modeling and simulations, where the "address" can be considered the "body" of the hydromorphone molecule and the "message" delivered by the substituent (tail) on the aromatic ring of the N-phenethyl moiety. One compound, N-p-chloro-phenethynorhydromorphone ((7aR,12bS)-3-(4-chlorophenethyl)-9-hydroxy-2,3,4,4a,5,6-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7(7aH)-one, 2i), was found to have nanomolar binding affinity at MOR and DOR. It was a potent partial agonist at MOR and a full potent agonist at DOR with a δ/μ potency ratio of 1.2 in the ([35S]GTPγS) assay. Bifunctional opioids that interact with MOR and DOR, the latter as agonists or antagonists, have been reported to have fewer side-effects than MOR agonists. The p-chlorophenethyl compound 2i was evaluated for its effect on respiration in both mice and squirrel monkeys. Compound 2i did not depress respiration (using normal air) in mice or squirrel monkeys. However, under conditions of hypercapnia (using air mixed with 5% CO2), respiration was depressed in squirrel monkeys.
Keywords: (−)-N-phenethylnorhydromorphone analogs; MOR and DOR agonists; [35S]GTPgammaS assay; bias factor; bifunctional ligands; forskolin-induced cAMP accumulation assays; molecular modeling & simulation; opioid; respiratory depression; β-arrestin recruitment assays.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Opioid ligands with mixed properties from substituted enantiomeric N-phenethyl-5-phenylmorphans. Synthesis of a micro-agonist delta-antagonist and delta-inverse agonists.Org Biomol Chem. 2007 Apr 21;5(8):1177-1190. doi: 10.1039/b618875c. Epub 2007 Mar 1. Org Biomol Chem. 2007. PMID: 17406716
-
Synthesis, modeling, and pharmacological evaluation of UMB 425, a mixed μ agonist/δ antagonist opioid analgesic with reduced tolerance liabilities.ACS Chem Neurosci. 2013 Sep 18;4(9):1256-66. doi: 10.1021/cn4000428. Epub 2013 Jun 11. ACS Chem Neurosci. 2013. PMID: 23713721 Free PMC article.
-
Development of novel LP1-based analogues with enhanced delta opioid receptor profile.Bioorg Med Chem. 2017 Sep 1;25(17):4745-4752. doi: 10.1016/j.bmc.2017.07.021. Epub 2017 Jul 11. Bioorg Med Chem. 2017. PMID: 28734666
-
Opioid ligands with mixed mu/delta opioid receptor interactions: an emerging approach to novel analgesics.AAPS J. 2006 Mar 10;8(1):E118-25. doi: 10.1208/aapsj080114. AAPS J. 2006. PMID: 16584118 Free PMC article. Review.
-
Bifunctional μ opioid and σ1 receptor ligands as novel analgesics with reduced side effects.Eur J Med Chem. 2021 Nov 5;223:113658. doi: 10.1016/j.ejmech.2021.113658. Epub 2021 Jun 18. Eur J Med Chem. 2021. PMID: 34175542 Review.
Cited by
-
Potent MOR Agonists from 2'-Hydroxy-5,9-dimethyl-N-phenethyl Substituted-6,7-benzomorphans and from C8-Hydroxy, Methylene and Methyl Derivatives of N-Phenethylnormetazocine.Molecules. 2023 Nov 22;28(23):7709. doi: 10.3390/molecules28237709. Molecules. 2023. PMID: 38067439 Free PMC article.
-
A Journey through Diastereomeric Space: The Design, Synthesis, In Vitro and In Vivo Pharmacological Activity, and Molecular Modeling of Novel Potent Diastereomeric MOR Agonists and Antagonists.Molecules. 2022 Sep 30;27(19):6455. doi: 10.3390/molecules27196455. Molecules. 2022. PMID: 36234992 Free PMC article.
-
Opioids and Their Receptors: Present and Emerging Concepts in Opioid Drug Discovery.Molecules. 2020 Dec 1;25(23):5658. doi: 10.3390/molecules25235658. Molecules. 2020. PMID: 33271753 Free PMC article.
-
Recent Chemical and Pharmacological Developments on 14-Oxygenated-N-methylmorphinan-6-ones.Molecules. 2021 Sep 18;26(18):5677. doi: 10.3390/molecules26185677. Molecules. 2021. PMID: 34577147 Free PMC article. Review.
-
The use of hypercapnic conditions to assess opioid-induced respiratory depression in rats.J Pharmacol Toxicol Methods. 2021 Sep-Oct;111:107101. doi: 10.1016/j.vascn.2021.107101. Epub 2021 Jul 7. J Pharmacol Toxicol Methods. 2021. PMID: 34242797 Free PMC article.
References
-
- Ben Haddou T., Béni S., Hosztafi S., Malfacini D., Calo G., Schmidhammer H., Spetea M. Pharmacological investigations of N-substituent variation in morphine and oxymorphone: Opioid receptor binding, signaling and antinociceptive activity. PLoS ONE. 2014;9:e99231. doi: 10.1371/journal.pone.0099231. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

