Mature oligodendrocytes bordering lesions limit demyelination and favor myelin repair via heparan sulfate production
- PMID: 32515730
- PMCID: PMC7308090
- DOI: 10.7554/eLife.51735
Mature oligodendrocytes bordering lesions limit demyelination and favor myelin repair via heparan sulfate production
Abstract
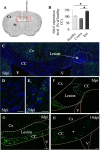
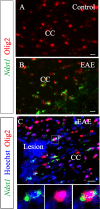
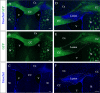
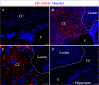
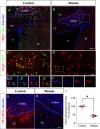
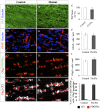
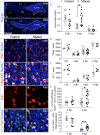
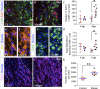
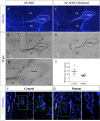
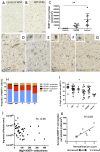
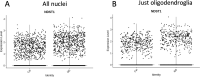
Myelin destruction is followed by resident glia activation and mobilization of endogenous progenitors (OPC) which participate in myelin repair. Here we show that in response to demyelination, mature oligodendrocytes (OLG) bordering the lesion express Ndst1, a key enzyme for heparan sulfates (HS) synthesis. Ndst1+ OLG form a belt that demarcates lesioned from intact white matter. Mice with selective inactivation of Ndst1 in the OLG lineage display increased lesion size, sustained microglia and OPC reactivity. HS production around the lesion allows Sonic hedgehog (Shh) binding and favors the local enrichment of this morphogen involved in myelin regeneration. In MS patients, Ndst1 is also found overexpressed in oligodendroglia and the number of Ndst1-expressing oligodendroglia is inversely correlated with lesion size and positively correlated with remyelination potential. Our study suggests that mature OLG surrounding demyelinated lesions are not passive witnesses but contribute to protection and regeneration by producing HS.
Keywords: Human; Ndst1; heparan sulfate; microglia; mouse; multiple sclerosis; myelin; neuroscience.
© 2020, Macchi et al.
Conflict of interest statement
MM, KM, CZ, EP, BE, BB, SJ, KG, FK, AW, MC, PD No competing interests declared
Figures


Similar articles
-
Myelin regulatory factor drives remyelination in multiple sclerosis.Acta Neuropathol. 2017 Sep;134(3):403-422. doi: 10.1007/s00401-017-1741-7. Epub 2017 Jun 19. Acta Neuropathol. 2017. PMID: 28631093
-
Sonic Hedgehog signaling is a positive oligodendrocyte regulator during demyelination.J Neurosci. 2013 Jan 30;33(5):1759-72. doi: 10.1523/JNEUROSCI.3334-12.2013. J Neurosci. 2013. PMID: 23365216 Free PMC article.
-
Genetic detection of Sonic hedgehog (Shh) expression and cellular response in the progression of acute through chronic demyelination and remyelination.Neurobiol Dis. 2018 Jul;115:145-156. doi: 10.1016/j.nbd.2018.04.003. Epub 2018 Apr 6. Neurobiol Dis. 2018. PMID: 29627579
-
The role of oligodendrocytes and oligodendrocyte progenitors in CNS remyelination.Adv Exp Med Biol. 1999;468:183-97. doi: 10.1007/978-1-4615-4685-6_15. Adv Exp Med Biol. 1999. PMID: 10635029 Review.
-
Oligodendrocytes and Microglia: Key Players in Myelin Development, Damage and Repair.Biomolecules. 2021 Jul 20;11(7):1058. doi: 10.3390/biom11071058. Biomolecules. 2021. PMID: 34356682 Free PMC article. Review.
Cited by
-
Myelin Repair: From Animal Models to Humans.Front Cell Neurosci. 2021 Apr 14;15:604865. doi: 10.3389/fncel.2021.604865. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33935649 Free PMC article. Review.
-
Distinct characteristics and severity of brain magnetic resonance imaging lesions in women and men with multiple sclerosis assessed using verified texture analysis measures.Front Neurol. 2023 Aug 10;14:1213377. doi: 10.3389/fneur.2023.1213377. eCollection 2023. Front Neurol. 2023. PMID: 37638198 Free PMC article.
-
Brief electrical nerve stimulation enhances intrinsic repair capacity of the focally demyelinated central nervous system.Neural Regen Res. 2022 May;17(5):1042-1050. doi: 10.4103/1673-5374.324848. Neural Regen Res. 2022. PMID: 34558531 Free PMC article.
-
Glial Cells as Key Regulators in Neuroinflammatory Mechanisms Associated with Multiple Sclerosis.Int J Mol Sci. 2024 Sep 4;25(17):9588. doi: 10.3390/ijms25179588. Int J Mol Sci. 2024. PMID: 39273535 Free PMC article. Review.
-
Heparanome-Mediated Rescue of Oligodendrocyte Progenitor Quiescence following Inflammatory Demyelination.J Neurosci. 2021 Mar 10;41(10):2245-2263. doi: 10.1523/JNEUROSCI.0580-20.2021. Epub 2021 Jan 20. J Neurosci. 2021. PMID: 33472827 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

