Advances in Small-Cell Lung Cancer (SCLC) Translational Research
- PMID: 32513672
- PMCID: PMC8015694
- DOI: 10.1101/cshperspect.a038240
Advances in Small-Cell Lung Cancer (SCLC) Translational Research
Abstract
Over the past several years, we have witnessed a resurgence of interest in the biology and therapeutic vulnerabilities of small-cell lung cancer (SCLC). This has been driven in part through the development of a more extensive array of representative models of disease, including a diverse variety of genetically engineered mouse models and human tumor xenografts. Herein, we review recent progress in SCLC model development, and consider some of the particularly active avenues of translational research in SCLC, including interrogation of intratumoral heterogeneity, insights into the cell of origin and oncogenic drivers, mechanisms of chemoresistance, and new therapeutic opportunities including biomarker-directed targeted therapies and immunotherapies. Whereas SCLC remains a highly lethal disease, these new avenues of translational research, bringing together mechanism-based preclinical and clinical research, offer new hope for patients with SCLC.
Copyright © 2021 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
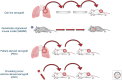
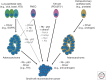

Similar articles
-
Genomic and pathological heterogeneity in clinically diagnosed small cell lung cancer in never/light smokers identifies therapeutically targetable alterations.Mol Oncol. 2021 Jan;15(1):27-42. doi: 10.1002/1878-0261.12673. Epub 2020 Nov 25. Mol Oncol. 2021. PMID: 32191822 Free PMC article.
-
MYC-Driven Small-Cell Lung Cancer is Metabolically Distinct and Vulnerable to Arginine Depletion.Clin Cancer Res. 2019 Aug 15;25(16):5107-5121. doi: 10.1158/1078-0432.CCR-18-4140. Epub 2019 Jun 4. Clin Cancer Res. 2019. PMID: 31164374 Free PMC article.
-
ESRP1 regulates alternative splicing of CARM1 to sensitize small cell lung cancer cells to chemotherapy by inhibiting TGF-β/Smad signaling.Aging (Albany NY). 2021 Jan 20;13(3):3554-3572. doi: 10.18632/aging.202295. Epub 2021 Jan 20. Aging (Albany NY). 2021. PMID: 33495408 Free PMC article.
-
Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data.Nat Rev Cancer. 2019 May;19(5):289-297. doi: 10.1038/s41568-019-0133-9. Nat Rev Cancer. 2019. PMID: 30926931 Free PMC article. Review.
-
Unravelling the biology of SCLC: implications for therapy.Nat Rev Clin Oncol. 2017 Sep;14(9):549-561. doi: 10.1038/nrclinonc.2017.71. Epub 2017 May 23. Nat Rev Clin Oncol. 2017. PMID: 28534531 Free PMC article. Review.
Cited by
-
Genetically-engineered mouse models of small cell lung cancer: the next generation.Oncogene. 2024 Feb;43(7):457-469. doi: 10.1038/s41388-023-02929-7. Epub 2024 Jan 8. Oncogene. 2024. PMID: 38191672 Free PMC article. Review.
-
Small-cell lung cancer.Nat Rev Dis Primers. 2021 Jan 14;7(1):3. doi: 10.1038/s41572-020-00235-0. Nat Rev Dis Primers. 2021. PMID: 33446664 Free PMC article. Review.
-
PTEN Loss Expands the Histopathologic Diversity and Lineage Plasticity of Lung Cancers Initiated by Rb1/Trp53 Deletion.J Thorac Oncol. 2023 Mar;18(3):324-338. doi: 10.1016/j.jtho.2022.11.019. Epub 2022 Dec 5. J Thorac Oncol. 2023. PMID: 36473627 Free PMC article.
-
Molecular subtyping of small-cell lung cancer based on mutational signatures with different genomic features and therapeutic strategies.Cancer Sci. 2023 Feb;114(2):665-679. doi: 10.1111/cas.15606. Epub 2022 Oct 28. Cancer Sci. 2023. PMID: 36178064 Free PMC article.
-
Efficacy and toxicity profile of first-line treatment for extensive-stage small cell lung cancer: A Bayesian network meta-analysis.Cancer Med. 2023 May;12(9):10230-10242. doi: 10.1002/cam4.5750. Epub 2023 Mar 23. Cancer Med. 2023. PMID: 36951654 Free PMC article.
References
-
- American Cancer Society. 2020. Cancer facts & figures. The American Cancer Society, Atlanta, GA. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts....
-
- Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, Jäger D, Pietanza MC, Le DT, de Braud F, et al. 2016. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 17: 883–895. 10.1016/S1470-2045(16)30098-5 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
