Chronic Sulforaphane Administration Inhibits Resistance to the mTOR-Inhibitor Everolimus in Bladder Cancer Cells
- PMID: 32512849
- PMCID: PMC7312500
- DOI: 10.3390/ijms21114026
Chronic Sulforaphane Administration Inhibits Resistance to the mTOR-Inhibitor Everolimus in Bladder Cancer Cells
Abstract
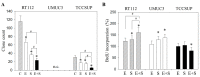
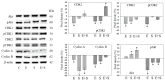
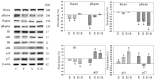
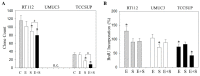
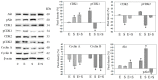
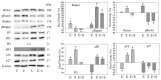
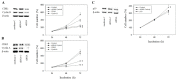
Progressive bladder cancer growth is associated with abnormal activation of the mammalian target of the rapamycin (mTOR) pathway, but treatment with an mTOR inhibitor has not been as effective as expected. Rather, resistance develops under chronic drug use, prompting many patients to lower their relapse risk by turning to natural, plant-derived products. The present study was designed to evaluate whether the natural compound, sulforaphane (SFN), combined with the mTOR inhibitor everolimus, could block the growth and proliferation of bladder cancer cells in the short- and long-term. The bladder cancer cell lines RT112, UMUC3, and TCCSUP were exposed short- (24 h) or long-term (8 weeks) to everolimus (0.5 nM) or SFN (2.5 µM) alone or in combination. Cell growth, proliferation, apoptosis, cell cycle progression, and cell cycle regulating proteins were evaluated. siRNA blockade was used to investigate the functional impact of the proteins. Short-term application of SFN and/or everolimus resulted in significant tumor growth suppression, with additive inhibition on clonogenic tumor growth. Long-term everolimus treatment resulted in resistance development characterized by continued growth, and was associated with elevated Akt-mTOR signaling and cyclin-dependent kinase (CDK)1 phosphorylation and down-regulation of p19 and p27. In contrast, SFN alone or SFN+everolimus reduced cell growth and proliferation. Akt and Rictor signaling remained low, and p19 and p27 expressions were high under combined drug treatment. Long-term exposure to SFN+everolimus also induced acetylation of the H3 and H4 histones. Phosphorylation of CDK1 was diminished, whereby down-regulation of CDK1 and its binding partner, Cyclin B, inhibited tumor growth. In conclusion, the addition of SFN to the long-term everolimus application inhibits resistance development in bladder cancer cells in vitro. Therefore, sulforaphane may hold potential for treating bladder carcinoma in patients with resistance to an mTOR inhibitor.
Keywords: bladder cancer; drug resistance; everolimus; growth; mTOR; proliferation; sulforaphane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
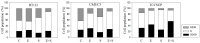

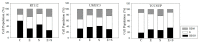
Similar articles
-
Bladder Cancer Metastasis Induced by Chronic Everolimus Application Can Be Counteracted by Sulforaphane In Vitro.Int J Mol Sci. 2020 Aug 4;21(15):5582. doi: 10.3390/ijms21155582. Int J Mol Sci. 2020. PMID: 32759798 Free PMC article.
-
Sulforaphane as an adjunctive to everolimus counteracts everolimus resistance in renal cancer cell lines.Phytomedicine. 2017 Apr 15;27:1-7. doi: 10.1016/j.phymed.2017.01.016. Epub 2017 Jan 31. Phytomedicine. 2017. PMID: 28314474
-
Sulforaphane inhibits proliferation and invasive activity of everolimus-resistant kidney cancer cells in vitro.Oncotarget. 2016 Dec 20;7(51):85208-85219. doi: 10.18632/oncotarget.13421. Oncotarget. 2016. PMID: 27863441 Free PMC article.
-
Role of mTOR in anticancer drug resistance: perspectives for improved drug treatment.Drug Resist Updat. 2008 Jun;11(3):63-76. doi: 10.1016/j.drup.2008.03.001. Epub 2008 Apr 28. Drug Resist Updat. 2008. PMID: 18440854 Free PMC article. Review.
-
Deficiency in the Treatment Description of mTOR Inhibitor Resistance in Medulloblastoma, a Systematic Review.Int J Mol Sci. 2021 Dec 31;23(1):464. doi: 10.3390/ijms23010464. Int J Mol Sci. 2021. PMID: 35008889 Free PMC article. Review.
Cited by
-
Bladder Cancer Metastasis Induced by Chronic Everolimus Application Can Be Counteracted by Sulforaphane In Vitro.Int J Mol Sci. 2020 Aug 4;21(15):5582. doi: 10.3390/ijms21155582. Int J Mol Sci. 2020. PMID: 32759798 Free PMC article.
-
Modulation of TLR/NF-κB/NLRP Signaling by Bioactive Phytocompounds: A Promising Strategy to Augment Cancer Chemotherapy and Immunotherapy.Front Oncol. 2022 Mar 1;12:834072. doi: 10.3389/fonc.2022.834072. eCollection 2022. Front Oncol. 2022. PMID: 35299751 Free PMC article.
-
The Emerging Role of Cyclin-Dependent Kinase Inhibitors in Treating Diet-Induced Obesity: New Opportunities for Breast and Ovarian Cancers?Cancers (Basel). 2022 May 30;14(11):2709. doi: 10.3390/cancers14112709. Cancers (Basel). 2022. PMID: 35681689 Free PMC article. Review.
-
Sulforaphane activates CD8+ T cells antitumor response through IL-12RB2/MMP3/FasL-induced MDSCs apoptosis'.J Immunother Cancer. 2024 Jan 31;12(1):e007983. doi: 10.1136/jitc-2023-007983. J Immunother Cancer. 2024. PMID: 38296593 Free PMC article.
-
Harnessing Sulforaphane Potential as a Chemosensitizing Agent: A Comprehensive Review.Cancers (Basel). 2024 Jan 5;16(2):244. doi: 10.3390/cancers16020244. Cancers (Basel). 2024. PMID: 38254735 Free PMC article. Review.
References
-
- Von Der Maase H., Sengelov L., Roberts J.T., Ricci S., Dogliotti L., Oliver T., Moore M.J., Zimmermann A., Arning M. Long-Term Survival Results of a Randomized Trial Comparing Gemcitabine Plus Cisplatin, with Methotrexate, Vinblastine, Doxorubicin, Plus Cisplatin in Patients With Bladder Cancer. J. Clin. Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. - DOI - PubMed
-
- Liu S.T., Hui G., Mathis C., Chamie K., Pantuck A.J., Drakaki A. The Current Status and Future Role of the Phosphoinositide 3 Kinase/AKT Signaling Pathway in Urothelial Cancer: An Old Pathway in the New Immunotherapy Era. Clin. Genitourin. Cancer. 2017;16:e269–e276. doi: 10.1016/j.clgc.2017.10.011. - DOI - PubMed
-
- Dalbagni G., Benfante N., Sjoberg D., Bochner B.H., Donat S.M., Herr H.W., Mc Coy A.S., Fahrner A.J., Retinger C., Rosenberg J.E., et al. Single Arm Phase I/II Study of Everolimus and Intravesical Gemcitabine in Patients with Primary or Secondary Carcinoma In Situ of the Bladder who failed Bacillus Calmette Guerin (NCT01259063) Bladder Cancer. 2017;3:113–119. doi: 10.3233/BLC-170095. - DOI - PMC - PubMed
-
- Pulido M., Roubaud G., Cazeau A.-L., Mahammedi H., Védrine L., Joly F., Mourey L., Pfister C., Goberna A., Lortal B., et al. Safety and efficacy of temsirolimus as second line treatment for patients with recurrent bladder cancer. BMC Cancer. 2018;18:194. doi: 10.1186/s12885-018-4059-5. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

