Mitochondrial transfer from mesenchymal stem cells improves neuronal metabolism after oxidant injury in vitro: The role of Miro1
- PMID: 32501156
- PMCID: PMC7983509
- DOI: 10.1177/0271678X20928147
Mitochondrial transfer from mesenchymal stem cells improves neuronal metabolism after oxidant injury in vitro: The role of Miro1
Abstract
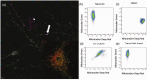
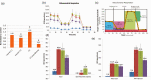
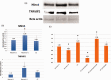
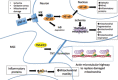
Stroke-induced cerebral ischemia is a major cause of death and disability. The disruption of blood flow results in neuronal and glial cell death leading to brain injury. Reperfusion restores oxygen to the affected tissue, but can also cause damage through an enhanced oxidative stress and inflammatory response. This study examines mitochondrial transfer from MSC to neurons and the role it plays in neuronal preservation after oxidant injury. We observed the transfer of mitochondria from MSC to mouse neurons in vitro following hydrogen peroxide exposure. The observed transfer was dependent on cell-to-cell contact and led to increased neuronal survival and improved metabolism. A number of pro-inflammatory and mitochondrial motility genes were upregulated in neurons after hydrogen peroxide exposure. This included Miro1 and TNFAIP2, linking inflammation and mitochondrial transfer to oxidant injury. Increasing Miro1 expression in MSC improved the metabolic benefit of mitochondrial transfer after neuronal oxidant injury. Decreasing Miro1 expression had the opposite effect, decreasing the metabolic benefit of MSC co-culture. MSC transfer of mitochondria to oxidant-damaged neurons may help improve neuronal preservation and functional recovery after stroke.
Keywords: Brain ischemia; Miro1; mesenchymal stem cell transplant; mitochondrial transfer; neuronal injury.
Conflict of interest statement
Figures

Similar articles
-
Neuroprotection by mesenchymal stem cell (MSC) administration is enhanced by local cooling infusion (LCI) in ischemia.Brain Res. 2019 Dec 1;1724:146406. doi: 10.1016/j.brainres.2019.146406. Epub 2019 Aug 24. Brain Res. 2019. PMID: 31454517 Review.
-
Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy.EMBO J. 2014 May 2;33(9):994-1010. doi: 10.1002/embj.201386030. Epub 2014 Jan 15. EMBO J. 2014. PMID: 24431222 Free PMC article.
-
Improving the Post-Stroke Therapeutic Potency of Mesenchymal Multipotent Stromal Cells by Cocultivation With Cortical Neurons: The Role of Crosstalk Between Cells.Stem Cells Transl Med. 2015 Sep;4(9):1011-20. doi: 10.5966/sctm.2015-0010. Epub 2015 Jul 9. Stem Cells Transl Med. 2015. PMID: 26160961 Free PMC article.
-
Miro1 Regulates Neuronal Mitochondrial Transport and Distribution to Alleviate Neuronal Damage in Secondary Brain Injury After Intracerebral Hemorrhage in Rats.Cell Mol Neurobiol. 2021 May;41(4):795-812. doi: 10.1007/s10571-020-00887-2. Epub 2020 Jun 4. Cell Mol Neurobiol. 2021. PMID: 32500352
-
Musculoskeletal Progenitor/Stromal Cell-Derived Mitochondria Modulate Cell Differentiation and Therapeutical Function.Front Immunol. 2021 Mar 8;12:606781. doi: 10.3389/fimmu.2021.606781. eCollection 2021. Front Immunol. 2021. PMID: 33763061 Free PMC article. Review.
Cited by
-
Key Role of Mesenchymal Stromal Cell Interaction with Macrophages in Promoting Repair of Lung Injury.Int J Mol Sci. 2023 Feb 8;24(4):3376. doi: 10.3390/ijms24043376. Int J Mol Sci. 2023. PMID: 36834784 Free PMC article. Review.
-
Potential mechanisms and therapeutic targets of mesenchymal stem cell transplantation for ischemic stroke.Stem Cell Res Ther. 2022 May 12;13(1):195. doi: 10.1186/s13287-022-02876-2. Stem Cell Res Ther. 2022. PMID: 35551643 Free PMC article. Review.
-
Oxidative Stress, Inflammation, and Autophagy: Potential Targets of Mesenchymal Stem Cells-Based Therapies in Ischemic Stroke.Front Neurosci. 2021 Feb 26;15:641157. doi: 10.3389/fnins.2021.641157. eCollection 2021. Front Neurosci. 2021. PMID: 33716657 Free PMC article. Review.
-
Headway and the remaining hurdles of mesenchymal stem cells therapy for bronchopulmonary dysplasia.Clin Respir J. 2022 Oct;16(10):629-645. doi: 10.1111/crj.13540. Epub 2022 Sep 2. Clin Respir J. 2022. PMID: 36055758 Free PMC article. Review.
-
Platelets Facilitate Wound Healing by Mitochondrial Transfer and Reducing Oxidative Stress in Endothelial Cells.Oxid Med Cell Longev. 2023 Feb 20;2023:2345279. doi: 10.1155/2023/2345279. eCollection 2023. Oxid Med Cell Longev. 2023. PMID: 36860732 Free PMC article.
References
-
- Horstmann A, Frisch S, Jentzsch R, et al.. Resuscitating the heart but losing the brain: brain atrophy in the aftermath of cardiac arrest. Neurology 2010; 74: 306–312. - PubMed
-
- Lim C, Alexander M, LaFleche G, et al.. The neurological and cognitive sequelae of cardiac arrest. Neurology 2004; 63: 1774–1778. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

