Effects of GH/IGF on the Aging Mitochondria
- PMID: 32498386
- PMCID: PMC7349719
- DOI: 10.3390/cells9061384
Effects of GH/IGF on the Aging Mitochondria
Abstract
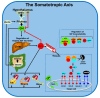
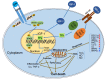
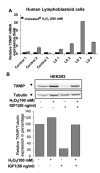
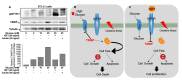
The mitochondria are key organelles regulating vital processes in the eukaryote cell. A decline in mitochondrial function is one of the hallmarks of aging. Growth hormone (GH) and the insulin-like growth factor-1 (IGF-1) are somatotropic hormones that regulate cellular homeostasis and play significant roles in cell differentiation, function, and survival. In mammals, these hormones peak during puberty and decline gradually during adulthood and aging. Here, we review the evidence that GH and IGF-1 regulate mitochondrial mass and function and contribute to specific processes of cellular aging. Specifically, we discuss the contribution of GH and IGF-1 to mitochondrial biogenesis, respiration and ATP production, oxidative stress, senescence, and apoptosis. Particular emphasis was placed on how these pathways intersect during aging.
Keywords: aging; growth hormone; insulin-like growth factor-1; mitochondria; oxidative stress; senescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Growth hormone, insulin-like growth factor-1 and the aging brain.Exp Gerontol. 2015 Aug;68:76-81. doi: 10.1016/j.exger.2014.10.002. Epub 2014 Oct 7. Exp Gerontol. 2015. PMID: 25300732 Free PMC article. Review.
-
Effects of growth hormone and insulin-like growth factor-1 on hepatocyte antioxidative enzymes.Exp Biol Med (Maywood). 2002 Feb;227(2):94-104. doi: 10.1177/153537020222700203. Exp Biol Med (Maywood). 2002. PMID: 11815672
-
Does the GH/IGF-1 axis contribute to skeletal sexual dimorphism? Evidence from mouse studies.Growth Horm IGF Res. 2016 Apr;27:7-17. doi: 10.1016/j.ghir.2015.12.004. Epub 2015 Dec 31. Growth Horm IGF Res. 2016. PMID: 26843472 Free PMC article. Review.
-
Effect of aging on growth hormone-induced insulin-like growth factor-I secretion from cultured rat chondrocytes.Growth Horm IGF Res. 1998 Oct;8(5):403-9. doi: 10.1016/s1096-6374(98)80311-0. Growth Horm IGF Res. 1998. PMID: 10984302
-
Effects of GH/IGF axis on bone and cartilage.Mol Cell Endocrinol. 2021 Jan 1;519:111052. doi: 10.1016/j.mce.2020.111052. Epub 2020 Oct 14. Mol Cell Endocrinol. 2021. PMID: 33068640 Free PMC article. Review.
Cited by
-
Insulin-Like Growth Factors in Development, Cancers and Aging.Cells. 2020 Oct 17;9(10):2309. doi: 10.3390/cells9102309. Cells. 2020. PMID: 33080771 Free PMC article.
-
The consequences of a high-calorie diet background before calorie restriction on skeletal muscles in a mouse model.Aging (Albany NY). 2021 Jun 24;13(12):16834-16858. doi: 10.18632/aging.203237. Epub 2021 Jun 24. Aging (Albany NY). 2021. PMID: 34166224 Free PMC article.
-
Vitamin D3 supplementation improves spatial memory, muscle function, pain score, and modulates different functional physiological biomarkers in vitamin D3 deficiency diet (VDD)-induced rats model.BMC Nutr. 2023 Sep 25;9(1):108. doi: 10.1186/s40795-023-00767-0. BMC Nutr. 2023. PMID: 37749664 Free PMC article.
-
Extending lifespan by modulating the growth hormone/insulin-like growth factor-1 axis: coming of age.Pituitary. 2021 Jun;24(3):438-456. doi: 10.1007/s11102-020-01117-0. Epub 2021 Jan 18. Pituitary. 2021. PMID: 33459974 Free PMC article. Review.
-
The Potential Role of Nutrition in Overtraining Syndrome: A Narrative Review.Nutrients. 2023 Nov 24;15(23):4916. doi: 10.3390/nu15234916. Nutrients. 2023. PMID: 38068774 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

