Human Cytomegalovirus Glycoprotein-Initiated Signaling Mediates the Aberrant Activation of Akt
- PMID: 32493823
- PMCID: PMC7394886
- DOI: 10.1128/JVI.00167-20
Human Cytomegalovirus Glycoprotein-Initiated Signaling Mediates the Aberrant Activation of Akt
Abstract
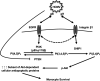
Human cytomegalovirus (HCMV) is a major cause of morbidity and mortality among immunocompromised and immunonaive individuals. HCMV-induced signaling initiated during viral entry stimulates a rapid noncanonical activation of Akt to drive the differentiation of short-lived monocytes into long-lived macrophages, which is essential for viral dissemination and persistence. We found that HCMV glycoproteins gB and gH directly bind and activate cellular epidermal growth factor receptor (EGFR) and integrin β1, respectively, to reshape canonical Akt signaling within monocytes. The remodeling of the Akt signaling network was due to the recruitment of nontraditional Akt activators to either the gB- or gH-generated receptor signaling complexes. Phosphoinositide 3-kinase (PI3K) comprised of the p110β catalytic subunit was recruited to the gB/EGFR complex despite p110δ being the primary PI3K isoform found within monocytes. Concomitantly, SH2 domain-containing inositol 5-phosphatase 1 (SHIP1) was recruited to the gH/integrin β1 complex, which is critical to aberrant Akt activation, as SHIP1 diverts PI3K signaling toward a noncanonical pathway. Although integrin β1 was required for SHIP1 recruitment, gB-activated EGFR mediated SHIP1 activation, underscoring the importance of the interplay between gB- and gH-mediated signaling to the unique activation of Akt during HCMV infection. Indeed, SHIP1 activation mediated the increased expression of Mcl-1 and HSP27, two Akt-dependent antiapoptotic proteins specifically upregulated during HCMV infection but not during growth factor treatment. Overall, our data indicate that HCMV glycoproteins gB and gH work in concert to initiate an HCMV-specific signalosome responsible for the atypical activation of Akt required for infected monocyte survival and ultimately viral persistence.IMPORTANCE Human cytomegalovirus (HCMV) infection is endemic throughout the world regardless of socioeconomic conditions and geographic locations with a seroprevalence reaching up to 100% in some developing countries. Although asymptomatic in healthy individuals, HCMV can cause severe multiorgan disease in immunocompromised or immunonaive patients. HCMV disease is a direct consequence of monocyte-mediated systematic spread of the virus following infection. Because monocytes are short-lived cells, HCMV must subvert the natural short life-span of these blood cells by inducing a distinct activation of Akt, a serine/theonine protein kinase. In this work, we demonstrate that HCMV glycoproteins gB and gH work in tandem to reroute classical host cellular receptor signaling to aberrantly activate Akt and drive survival of infected monocytes. Deciphering how HCMV modulates the cellular pathway to induce monocyte survival is important to develop a new class of anti-HCMV drugs that could target and prevent spread of the virus by eliminating infected monocytes.
Keywords: cytomegalovirus; monocytes.
Copyright © 2020 American Society for Microbiology.
Figures
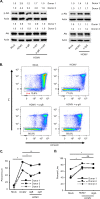
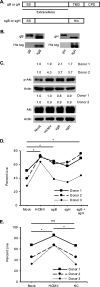

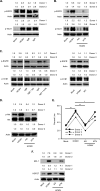
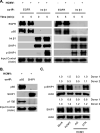

Similar articles
-
Human cytomegalovirus modulates mTORC1 to redirect mRNA translation within quiescently infected monocytes.J Virol. 2024 Feb 20;98(2):e0188823. doi: 10.1128/jvi.01888-23. Epub 2024 Jan 30. J Virol. 2024. PMID: 38289104 Free PMC article.
-
Human Cytomegalovirus Induces an Atypical Activation of Akt To Stimulate the Survival of Short-Lived Monocytes.J Virol. 2016 Jun 24;90(14):6443-6452. doi: 10.1128/JVI.00214-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27147739 Free PMC article.
-
Human Cytomegalovirus Stimulates the Synthesis of Select Akt-Dependent Antiapoptotic Proteins during Viral Entry To Promote Survival of Infected Monocytes.J Virol. 2016 Jan 6;90(6):3138-47. doi: 10.1128/JVI.02879-15. J Virol. 2016. PMID: 26739047 Free PMC article.
-
Human cytomegalovirus induction of a unique signalsome during viral entry into monocytes mediates distinct functional changes: a strategy for viral dissemination.J Leukoc Biol. 2012 Oct;92(4):743-52. doi: 10.1189/jlb.0112040. Epub 2012 Jun 19. J Leukoc Biol. 2012. PMID: 22715139 Free PMC article. Review.
-
Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism.Viruses. 2018 Dec 11;10(12):704. doi: 10.3390/v10120704. Viruses. 2018. PMID: 30544948 Free PMC article. Review.
Cited by
-
Genome-Wide Meta-Analysis Identifies Multiple Novel Rare Variants to Predict Common Human Infectious Diseases Risk.Int J Mol Sci. 2023 Apr 10;24(8):7006. doi: 10.3390/ijms24087006. Int J Mol Sci. 2023. PMID: 37108169 Free PMC article.
-
Delivery of US28 by incoming HCMV particles rapidly attenuates Akt activity to suppress HCMV lytic replication in monocytes.Sci Signal. 2024 Aug 27;17(851):eadn8727. doi: 10.1126/scisignal.adn8727. Epub 2024 Aug 27. Sci Signal. 2024. PMID: 39190708 Free PMC article.
-
Human cytomegalovirus modulates mTORC1 to redirect mRNA translation within quiescently infected monocytes.J Virol. 2024 Feb 20;98(2):e0188823. doi: 10.1128/jvi.01888-23. Epub 2024 Jan 30. J Virol. 2024. PMID: 38289104 Free PMC article.
-
Human Cytomegalovirus UL7, miR-US5-1, and miR-UL112-3p Inactivation of FOXO3a Protects CD34+ Hematopoietic Progenitor Cells from Apoptosis.mSphere. 2021 Jan 6;6(1):e00986-20. doi: 10.1128/mSphere.00986-20. mSphere. 2021. PMID: 33408225 Free PMC article.
-
Mechanisms of Survival of Cytomegalovirus-Infected Tumor Cells.Mol Biol. 2022;56(5):668-683. doi: 10.1134/S0026893322050132. Epub 2022 Oct 5. Mol Biol. 2022. PMID: 36217337 Free PMC article.
References
-
- Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, Nabors LB, Cobbs CG, Britt WJ. 2002. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res 62:3347–3350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

