Sex-steroid-dependent plasticity of brain-stem autonomic circuits
- PMID: 32493037
- PMCID: PMC7468793
- DOI: 10.1152/ajpregu.00357.2019
Sex-steroid-dependent plasticity of brain-stem autonomic circuits
Abstract
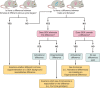
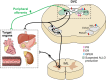
In the central nervous system (CNS), nuclei of the brain stem play a critical role in the integration of peripheral sensory information and the regulation of autonomic output in mammalian physiology. The nucleus tractus solitarius of the brain stem acts as a relay center that receives peripheral sensory input from vagal afferents of the nodose ganglia, integrates information from within the brain stem and higher central centers, and then transmits autonomic efferent output through downstream premotor nuclei, such as the nucleus ambiguus, the dorsal motor nucleus of the vagus, and the rostral ventral lateral medulla. Although there is mounting evidence that sex and sex hormones modulate autonomic physiology at the level of the CNS, the mechanisms and neurocircuitry involved in producing these functional consequences are poorly understood. Of particular interest in this review is the role of estrogen, progesterone, and 5α-reductase-dependent neurosteroid metabolites of progesterone (e.g., allopregnanolone) in the modulation of neurotransmission within brain-stem autonomic neurocircuits. This review will discuss our understanding of the actions and mechanisms of estrogen, progesterone, and neurosteroids at the cellular level of brain-stem nuclei. Understanding the complex interaction between sex hormones and neural signaling plasticity of the autonomic nervous system is essential to elucidating the role of sex in overall physiology and disease.
Keywords: autonomic; estrogen; neurosteroid; progesterone; sex.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
Similar articles
-
Brainstem circuits regulating gastric function.Annu Rev Physiol. 2006;68:279-305. doi: 10.1146/annurev.physiol.68.040504.094635. Annu Rev Physiol. 2006. PMID: 16460274 Free PMC article. Review.
-
Functional anatomy of the vagus system - Emphasis on the somato-visceral interface.Auton Neurosci. 2021 Dec;236:102887. doi: 10.1016/j.autneu.2021.102887. Epub 2021 Sep 28. Auton Neurosci. 2021. PMID: 34634680 Free PMC article.
-
Distributed actions and dynamic associations in respiratory-related neuronal assemblies of the ventrolateral medulla and brain stem midline: evidence from spike train analysis.J Neurophysiol. 1994 Oct;72(4):1830-51. doi: 10.1152/jn.1994.72.4.1830. J Neurophysiol. 1994. PMID: 7823104
-
Functional anatomy of the vagus system: How does the polyvagal theory comply?Biol Psychol. 2022 Oct;174:108425. doi: 10.1016/j.biopsycho.2022.108425. Epub 2022 Sep 12. Biol Psychol. 2022. PMID: 36100134 Review.
-
Vestibular nucleus projections to nucleus tractus solitarius and the dorsal motor nucleus of the vagus nerve: potential substrates for vestibulo-autonomic interactions.Exp Brain Res. 1994;98(2):200-12. doi: 10.1007/BF00228409. Exp Brain Res. 1994. PMID: 8050507
Cited by
-
Women's mood at high altitude. sexual dimorphism in hypoxic stress modulation by the tryptophan-melatonin axis.Front Physiol. 2023 Jan 17;13:1099276. doi: 10.3389/fphys.2022.1099276. eCollection 2022. Front Physiol. 2023. PMID: 36733695 Free PMC article. Review.
-
Hypoxia Differentially Affects Healthy Men and Women During a Daytime Nap With a Dose-Response Relationship: a Randomized, Cross-Over Pilot Study.Front Physiol. 2022 May 24;13:899636. doi: 10.3389/fphys.2022.899636. eCollection 2022. Front Physiol. 2022. PMID: 35685284 Free PMC article.
-
Regulation of Bone by Mechanical Loading, Sex Hormones, and Nerves: Integration of Such Regulatory Complexity and Implications for Bone Loss during Space Flight and Post-Menopausal Osteoporosis.Biomolecules. 2023 Jul 15;13(7):1136. doi: 10.3390/biom13071136. Biomolecules. 2023. PMID: 37509172 Free PMC article. Review.
-
Stress Adaptation and the Brainstem with Focus on Corticotropin-Releasing Hormone.Int J Mol Sci. 2021 Aug 23;22(16):9090. doi: 10.3390/ijms22169090. Int J Mol Sci. 2021. PMID: 34445795 Free PMC article. Review.
References
-
- Abramian AM, Comenencia-Ortiz E, Modgil A, Vien TN, Nakamura Y, Moore YE, Maguire JL, Terunuma M, Davies PA, Moss SJ. Neurosteroids promote phosphorylation and membrane insertion of extrasynaptic GABAA receptors. Proc Natl Acad Sci USA 111: 7132–7137, 2014. doi:10.1073/pnas.1403285111. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

