PuraMatrix hydrogel enhances the expression of motor neuron progenitor marker and improves adhesion and proliferation of motor neuron-like cells
- PMID: 32489557
- PMCID: PMC7239419
- DOI: 10.22038/ijbms.2020.39797.9434
PuraMatrix hydrogel enhances the expression of motor neuron progenitor marker and improves adhesion and proliferation of motor neuron-like cells
Abstract
Objectives: Cell therapy has provided clinical applications to the treatment of motor neuron diseases. The current obstacle in stem cell therapy is to direct differentiation of stem cells into neurons in the neurodegenerative disorders. Biomaterial scaffolds can improve cell differentiation and are widely used in translational medicine and tissue engineering. The aim of this study was to compare the efficiency of two-dimensional with a three-dimensional culture system in their ability to generate functional motor neuron-like cells from adipose-derived stem cells.
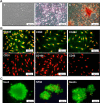
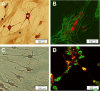
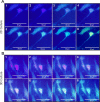
Materials and methods: We compared motor neuron-like cells derived from rat adipose tissue in differentiation, adhesion, proliferation, and functional properties on two-dimensional with three-dimensional culture systems. Neural differentiation was analyzed by immunocytochemistry for immature (Islet1) and mature (HB9, ChAT, and synaptophysin) motor neuron markers.
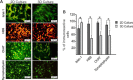
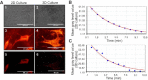
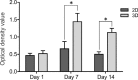
Results: Our results indicated that the three-dimensional environment exhibited an increase in the number of Islet1. In contrast, two-dimensional culture system resulted in more homeobox gene (HB9), Choline Acetyltransferase (ChAT), and synaptophysin positive cells. The results of this investigation showed that proliferation and adhesion of motor neuron-like cells significantly increased in three-dimensional compared with two-dimensional environments.
Conclusion: The findings of this study suggested that three-dimension may create a proliferative niche for motor neuron-like cells. Overall, this study strengthens the idea that three-dimensional culture may mimic neural stem cell environment for neural tissue regeneration.
Keywords: Motor neuron-like cells; Nanoscaffolds; Proliferation; Stem cell therapy; Three-dimension culture; Tissue engineering.
Figures



Similar articles
-
Differentiation of Wharton's Jelly-Derived Mesenchymal Stem Cells into Motor Neuron-Like Cells on Three-Dimensional Collagen-Grafted Nanofibers.Mol Neurobiol. 2016 May;53(4):2397-408. doi: 10.1007/s12035-015-9199-x. Epub 2015 May 24. Mol Neurobiol. 2016. PMID: 26001761
-
Rapid, efficient, and simple motor neuron differentiation from human pluripotent stem cells.Mol Brain. 2015 Dec 1;8(1):79. doi: 10.1186/s13041-015-0172-4. Mol Brain. 2015. PMID: 26626025 Free PMC article.
-
New methods for inducing the differentiation of amniotic-derived mesenchymal stem cells into motor neuron precursor cells.Tissue Cell. 2013 Oct;45(5):295-305. doi: 10.1016/j.tice.2013.03.002. Epub 2013 Jun 24. Tissue Cell. 2013. PMID: 23806299
-
Composition and Mechanism of Three-Dimensional Hydrogel System in Regulating Stem Cell Fate.Tissue Eng Part B Rev. 2020 Dec;26(6):498-518. doi: 10.1089/ten.TEB.2020.0021. Epub 2020 May 26. Tissue Eng Part B Rev. 2020. PMID: 32272868 Review.
-
Motor neuron replacement therapy for amyotrophic lateral sclerosis.Neural Regen Res. 2022 Aug;17(8):1633-1639. doi: 10.4103/1673-5374.332123. Neural Regen Res. 2022. PMID: 35017408 Free PMC article. Review.
Cited by
-
Neurorepair and Regeneration of the Brain: A Decade of Bioscaffolds and Engineered Microtissue.Front Cell Dev Biol. 2021 Apr 7;9:649891. doi: 10.3389/fcell.2021.649891. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33898443 Free PMC article. Review.
-
Directional induction of neural stem cells, a new therapy for neurodegenerative diseases and ischemic stroke.Cell Death Discov. 2023 Jul 1;9(1):215. doi: 10.1038/s41420-023-01532-9. Cell Death Discov. 2023. PMID: 37393356 Free PMC article. Review.
-
Sources, Characteristics, and Therapeutic Applications of Mesenchymal Cells in Tissue Engineering.Tissue Eng Regen Med. 2022 Apr;19(2):325-361. doi: 10.1007/s13770-021-00417-1. Epub 2022 Jan 29. Tissue Eng Regen Med. 2022. PMID: 35092596 Free PMC article. Review.
-
Combined treatment of high-intensity interval training with neural stem cell generation on contusive model of spinal cord injury in rats.Brain Behav. 2023 Jul;13(7):e3043. doi: 10.1002/brb3.3043. Epub 2023 May 11. Brain Behav. 2023. PMID: 37165750 Free PMC article.
References
-
- Pettikiriarachchi JT, Parish CL, Shoichet MS, Forsythe JS, Nisbet DR. Biomaterials for brain tissue engineering. Aust J Chem. 2010;63:1143–1154.
-
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. - PubMed
-
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
