Generation of Multipotent Stem Cells from Adult Human Peripheral Blood Following the Treatment with Platelet-Derived Mitochondria
- PMID: 32485922
- PMCID: PMC7349571
- DOI: 10.3390/cells9061350
Generation of Multipotent Stem Cells from Adult Human Peripheral Blood Following the Treatment with Platelet-Derived Mitochondria
Abstract
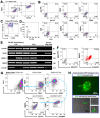
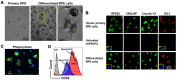
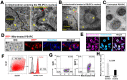
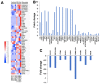
Autologous stem cells are highly preferred for cellular therapy to treat human diseases. Mitochondria are organelles normally located in cytoplasm. Our recent studies demonstrated the differentiation of adult peripheral blood-derived insulin-producing cells (designated PB-IPC) into hematopoietic-like cells after the treatment with platelet-derived mitochondria. To further explore the molecular mechanism and their therapeutic potentials, through confocal and electron microscopy, we found that mitochondria enter cells and directly penetrate the nucleus of PB-IPC after the treatment with platelet-derived mitochondria, where they can produce profound epigenetic changes as demonstrated by RNA-seq and PCR array. Ex vivo functional studies established that mitochondrion-induced PB-IPC (miPB-IPC) can give rise to retinal pigment epithelium (RPE) cells and neuronal cells in the presence of different inducers. Further colony analysis highlighted the multipotent capability of the differentiation of PB-IPC into three-germ layer-derived cells. Therefore, these data indicate a novel function of mitochondria in cellular reprogramming, leading to the generation of autologous multipotent stem cells for clinical applications.
Keywords: PB-IPC; cell reprogramming; differentiation; mitochondria; multipotent stem cells; platelets.
Conflict of interest statement
Dr. Zhao is a founder of Tianhe Stem Cell Biotechnology Inc. Dr. Zhao is an inventor of Stem Cell Educator technology and an inventor for technologies on platelets and mitochondria that have been submitted for patent applications (US 15/688 464, US 15/688 498). This work has been submitted for a provisional patent application. Y.Z, H.Y, W.H. and X.S. were listed as inventors.
Figures



Similar articles
-
Increase in the Expression of Glucose Transporter 2 (GLUT2) on the Peripheral Blood Insulin-Producing Cells (PB-IPC) in Type 1 Diabetic Patients after Receiving Stem Cell Educator Therapy.Int J Mol Sci. 2024 Jul 30;25(15):8337. doi: 10.3390/ijms25158337. Int J Mol Sci. 2024. PMID: 39125908 Free PMC article.
-
Notch-HEY2 signaling pathway contributes to the differentiation of CD34+ hematopoietic-like stem cells from adult peripheral blood insulin-producing cells after the treatment with platelet-derived mitochondria.Mol Biol Rep. 2020 Oct;47(10):8347-8352. doi: 10.1007/s11033-020-05874-w. Epub 2020 Sep 30. Mol Biol Rep. 2020. PMID: 32997309
-
Generation of Hematopoietic-Like Stem Cells from Adult Human Peripheral Blood Following Treatment with Platelet-Derived Mitochondria.Int J Mol Sci. 2020 Jun 15;21(12):4249. doi: 10.3390/ijms21124249. Int J Mol Sci. 2020. PMID: 32549211 Free PMC article.
-
Hematopoietic potential of neural stem cells: plasticity versus heterogeneity.Leuk Lymphoma. 2002 Dec;43(12):2263-8. doi: 10.1080/1042819021000040215. Leuk Lymphoma. 2002. PMID: 12613511 Review.
-
From bone marrow to therapeutic applications: different behaviour and genetic/epigenetic stability during mesenchymal stem cell expansion in autologous and foetal bovine sera?Int J Dev Biol. 2008;52(8):1023-32. doi: 10.1387/ijdb.082725gt. Int J Dev Biol. 2008. PMID: 18956335 Review.
Cited by
-
Effect of Thyroxine on the Structural and Dynamic Features of Cardiac Mitochondria and Mitophagy in Rats.Cells. 2023 Jan 21;12(3):396. doi: 10.3390/cells12030396. Cells. 2023. PMID: 36766738 Free PMC article.
-
Increase in the Expression of Glucose Transporter 2 (GLUT2) on the Peripheral Blood Insulin-Producing Cells (PB-IPC) in Type 1 Diabetic Patients after Receiving Stem Cell Educator Therapy.Int J Mol Sci. 2024 Jul 30;25(15):8337. doi: 10.3390/ijms25158337. Int J Mol Sci. 2024. PMID: 39125908 Free PMC article.
-
Immune Modulation of Platelet-Derived Mitochondria on Memory CD4+ T Cells in Humans.Int J Mol Sci. 2020 Aug 31;21(17):6295. doi: 10.3390/ijms21176295. Int J Mol Sci. 2020. PMID: 32878069 Free PMC article.
-
Mitochondria Transfer in Bone Marrow Hematopoietic Activity.Curr Stem Cell Rep. 2021 Mar;7(1):1-12. doi: 10.1007/s40778-020-00185-z. Epub 2021 Jan 14. Curr Stem Cell Rep. 2021. PMID: 36846725 Free PMC article.
-
Revisiting the Pathogenesis of Type 1 Diabetes: Importance of Neural Input to Pancreatic Islets and the Therapeutic Capability of Stem Cell Educator TM Therapy to Restore Their Integrity.Biomedicines. 2023 Feb 16;11(2):594. doi: 10.3390/biomedicines11020594. Biomedicines. 2023. PMID: 36831130 Free PMC article. Review.
References
-
- Lotfy M., Adeghate J., Kalasz H., Singh J., Adeghate E. Chronic Complications of Diabetes Mellitus: A Mini Review. Curr. Diabetes Rev. 2017;13:3–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

