Mouse intestinal tuft cells express advillin but not villin
- PMID: 32483224
- PMCID: PMC7264147
- DOI: 10.1038/s41598-020-65469-0
Mouse intestinal tuft cells express advillin but not villin
Abstract
Tuft (or brush) cells are solitary chemosensory cells scattered throughout the epithelia of the respiratory and alimentary tract. The actin-binding protein villin (Vil1) is used as a marker of tuft cells and the villin promoter is frequently used to drive expression of the Cre recombinase in tuft cells. While there is widespread agreement about the expression of villin in tuft cells there are several disagreements related to tuft cell lineage commitment and function. We now show that many of these inconsistencies could be resolved by our surprising finding that intestinal tuft cells, in fact, do not express villin protein. Furthermore, we show that a related actin-binding protein, advillin which shares 75% homology with villin, has a tuft cell restricted expression in the gastrointestinal epithelium. Our study identifies advillin as a marker of tuft cells and provides a mechanism for driving gene expression in tuft cells but not in other epithelial cells of the gastrointestinal tract. Our findings fundamentally change the way we identify and study intestinal tuft cells.
Conflict of interest statement
The authors declare no competing interests.
Figures

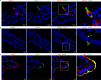

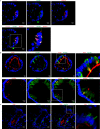

Similar articles
-
Advillin is a tuft cell marker in the mouse alimentary tract.J Mol Histol. 2020 Aug;51(4):421-435. doi: 10.1007/s10735-020-09893-6. Epub 2020 Jul 2. J Mol Histol. 2020. PMID: 32617896 Free PMC article.
-
Distribution pattern and molecular signature of cholinergic tuft cells in human gastro-intestinal and pancreatic-biliary tract.Sci Rep. 2019 Nov 25;9(1):17466. doi: 10.1038/s41598-019-53997-3. Sci Rep. 2019. PMID: 31767912 Free PMC article.
-
Villin-1 and Gelsolin Regulate Changes in Actin Dynamics That Affect Cell Survival Signaling Pathways and Intestinal Inflammation.Gastroenterology. 2018 Apr;154(5):1405-1420.e2. doi: 10.1053/j.gastro.2017.12.016. Epub 2017 Dec 21. Gastroenterology. 2018. PMID: 29274870 Free PMC article.
-
From the structure to the function of villin, an actin-binding protein of the brush border.Bioessays. 1990 Sep;12(9):403-8. doi: 10.1002/bies.950120902. Bioessays. 1990. PMID: 2256904 Review.
-
Epithelial cell growth and differentiation. IV. Controlled spatiotemporal expression of transgenes: new tools to study normal and pathological states.Am J Physiol. 1997 Oct;273(4):G759-62. doi: 10.1152/ajpgi.1997.273.4.G759. Am J Physiol. 1997. PMID: 9357815 Review.
Cited by
-
Tuft Cells Increase Following Ovine Intestinal Parasite Infections and Define Evolutionarily Conserved and Divergent Responses.Front Immunol. 2021 Nov 22;12:781108. doi: 10.3389/fimmu.2021.781108. eCollection 2021. Front Immunol. 2021. PMID: 34880874 Free PMC article.
-
Intestinal Tuft Cells Are Enriched With Protocadherins.J Histochem Cytochem. 2024 Oct;72(10):611-622. doi: 10.1369/00221554241287267. Epub 2024 Oct 3. J Histochem Cytochem. 2024. PMID: 39360911
-
Luminal Chemosensory Cells in the Small Intestine.Nutrients. 2021 Oct 22;13(11):3712. doi: 10.3390/nu13113712. Nutrients. 2021. PMID: 34835968 Free PMC article. Review.
-
Organization of a cytoskeletal superstructure in the apical domain of intestinal tuft cells.J Cell Biol. 2024 Dec 2;223(12):e202404070. doi: 10.1083/jcb.202404070. Epub 2024 Oct 1. J Cell Biol. 2024. PMID: 39352498 Free PMC article.
-
Sensory nerves in the spotlight of the stem cell niche.Stem Cells Transl Med. 2021 Mar;10(3):346-356. doi: 10.1002/sctm.20-0284. Epub 2020 Oct 28. Stem Cells Transl Med. 2021. PMID: 33112056 Free PMC article. Review.
References
-
- Von Moltke J. Intestinal tuft cells. Physiology of the Gastrointestinal Tract. 2018;Chapter 31:721–733. doi: 10.1016/B978-0-12-809954-4.00031-1. - DOI
-
- Isomaki AM. A new cell type (tuft cell) in the gastrointestinal mucosa of the rat. A transmission and scanning electron microscopic study. Acta Pathol Microbiol Scand A. 1973;Suppl 240:241–235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

