Activation of TWIST Transcription by Chromatin Remodeling Protein BRG1 Contributes to Liver Fibrosis in Mice
- PMID: 32478075
- PMCID: PMC7237740
- DOI: 10.3389/fcell.2020.00340
Activation of TWIST Transcription by Chromatin Remodeling Protein BRG1 Contributes to Liver Fibrosis in Mice
Abstract
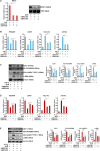
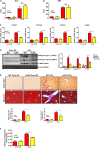
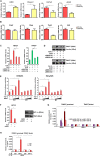
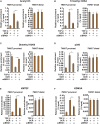
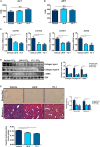
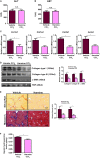
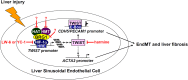
Liver fibrosis is a complex pathophysiological process to which many different cell types contribute. Endothelial cells play versatile roles in the regulation of liver fibrosis. The underlying epigenetic mechanism is not fully appreciated. In the present study, we investigated the role of BRG1, a chromatin remodeling protein, in the modulation of endothelial cells in response to pro-fibrogenic stimuli in vitro and liver fibrosis in mice. We report that depletion of BRG1 by siRNA abrogated TGF-β or hypoxia induced down-regulation of endothelial marker genes and up-regulation of mesenchymal marker genes in cultured endothelial cells. Importantly, endothelial-specific BRG1 deletion attenuated CCl4 induced liver fibrosis in mice. BRG1 knockdown in vitro or BRG1 knockout in vivo was accompanied by the down-regulation of TWIST, a key regulator of endothelial phenotype. Mechanistically, BRG1 interacted with and was recruited to the TWIST promoter by HIF-1α to activate TWIST transcription. BRG1 silencing rendered a more repressive chromatin structure surrounding the TWIST promoter likely contributing to TWIST down-regulation. Inhibition of HIF-1α activity dampened liver fibrosis in mice. Similarly, pharmaceutical inhibition of TWIST alleviated liver fibrosis in mice. In conclusion, our data suggest that epigenetic activation of TWIST by BRG1 contributes to the modulation of endothelial phenotype and liver fibrosis. Therefore, targeting the HIF1α-BRG1-TWIST axis may yield novel therapeutic solutions to treat liver fibrosis.
Keywords: Brg1; endothelial cell; epigenetics; liver fibrosis; transcriptional regulation.
Copyright © 2020 Dong, Kong, Zhu, Shao, Wu, Lu, Guo and Xu.
Figures
Similar articles
-
The Chromatin Remodeler Brg1 Integrates ROS Production and Endothelial-Mesenchymal Transition to Promote Liver Fibrosis in Mice.Front Cell Dev Biol. 2019 Oct 23;7:245. doi: 10.3389/fcell.2019.00245. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31750301 Free PMC article.
-
Dual roles of chromatin remodeling protein BRG1 in angiotensin II-induced endothelial-mesenchymal transition.Cell Death Dis. 2020 Jul 18;11(7):549. doi: 10.1038/s41419-020-02744-y. Cell Death Dis. 2020. PMID: 32683412 Free PMC article.
-
BRG1 deficiency in endothelial cells alleviates thioacetamide induced liver fibrosis in mice.Biochem Biophys Res Commun. 2020 Jan 1;521(1):212-219. doi: 10.1016/j.bbrc.2019.10.109. Epub 2019 Oct 18. Biochem Biophys Res Commun. 2020. PMID: 31635808
-
BRG1 regulates endothelial-derived IL-33 to promote ischemia-reperfusion induced renal injury and fibrosis in mice.Biochim Biophys Acta Mol Basis Dis. 2019 Sep 1;1865(9):2551-2561. doi: 10.1016/j.bbadis.2019.06.015. Epub 2019 Jun 19. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 31228616
-
Activation of Galectin-3 (LGALS3) Transcription by Injurious Stimuli in the Liver Is Commonly Mediated by BRG1.Front Cell Dev Biol. 2019 Nov 26;7:310. doi: 10.3389/fcell.2019.00310. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31850346 Free PMC article.
Cited by
-
BRG1 Links TLR4 Trans-Activation to LPS-Induced SREBP1a Expression and Liver Injury.Front Cell Dev Biol. 2021 Mar 18;9:617073. doi: 10.3389/fcell.2021.617073. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33816466 Free PMC article.
-
Epigenetics as a versatile regulator of fibrosis.J Transl Med. 2023 Mar 2;21(1):164. doi: 10.1186/s12967-023-04018-5. J Transl Med. 2023. PMID: 36864460 Free PMC article. Review.
-
Activation of TC10-Like Transcription by Lysine Demethylase KDM4B in Colorectal Cancer Cells.Front Cell Dev Biol. 2021 Jun 23;9:617549. doi: 10.3389/fcell.2021.617549. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34249900 Free PMC article.
-
Epiregulin (EREG) and Myocardin Related Transcription Factor A (MRTF-A) Form a Feedforward Loop to Drive Hepatic Stellate Cell Activation.Front Cell Dev Biol. 2021 Jan 15;8:591246. doi: 10.3389/fcell.2020.591246. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33520984 Free PMC article.
-
BRG1 Mediates Nephronectin Activation in Hepatocytes to Promote T Lymphocyte Infiltration in ConA-Induced Hepatitis.Front Cell Dev Biol. 2021 Jan 21;8:587502. doi: 10.3389/fcell.2020.587502. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33553140 Free PMC article.
References
-
- Brenner D. A., Waterboer T., Choi S. K., Lindquist J. N., Stefanovic B., Burchardt E., et al. (2000). New aspects of hepatic fibrosis. J. Hepatol.32 1(Suppl), 32–38. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

