Rapamycin treatment correlates changes in primary cilia expression with cell cycle regulation in epithelial cells
- PMID: 32470549
- PMCID: PMC7899243
- DOI: 10.1016/j.bcp.2020.114056
Rapamycin treatment correlates changes in primary cilia expression with cell cycle regulation in epithelial cells
Abstract
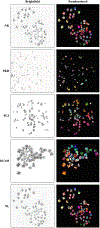
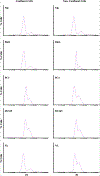
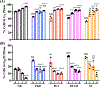
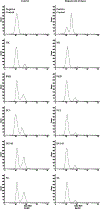
Primary cilia are sensory organelles that regulate cell cycle and signaling pathways. In addition to its association with cancer, dysfunction of primary cilia is responsible for the pathogenesis of polycystic kidney disease (PKD) and other ciliopathies. Because the association between cilia formation or length and cell cycle or division is poorly understood, we here evaluated their correlation in this study. Using Spectral Karyotyping (SKY) technique, we showed that PKD and the cancer/tumorigenic epithelial cells PC3, DU145, and NL20-TA were associated with abnormal ploidy. We also showed that PKD and the cancer epithelia were highly proliferative. Importantly, the cancer epithelial cells had a reduction in the presence and/or length of primary cilia relative to the normal kidney (NK) cells. We then used rapamycin to restore the expression and length of primary cilia in these cells. Our subsequent analyses indicated that both the presence and length of primary cilia were inversely correlated with cell proliferation. Collectively, our data suggest that restoring the presence and/or length of primary cilia may serve as a novel approach to inhibit cancer cell proliferation.
Keywords: Cancer; Karyotyping; Polycystic kidney disease; Proliferation; Wnt signalling.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


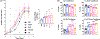

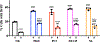





Similar articles
-
Inpp5e suppresses polycystic kidney disease via inhibition of PI3K/Akt-dependent mTORC1 signaling.Hum Mol Genet. 2016 Jun 1;25(11):2295-2313. doi: 10.1093/hmg/ddw097. Epub 2016 Apr 7. Hum Mol Genet. 2016. PMID: 27056978
-
Primary cilia and signaling pathways in mammalian development, health and disease.Nephron Physiol. 2009;111(3):p39-53. doi: 10.1159/000208212. Epub 2009 Mar 10. Nephron Physiol. 2009. PMID: 19276629 Free PMC article. Review.
-
Epigenetically Mediated Ciliogenesis and Cell Cycle Regulation, and Their Translational Potential.Cells. 2021 Jul 2;10(7):1662. doi: 10.3390/cells10071662. Cells. 2021. PMID: 34359832 Free PMC article. Review.
-
RAPAMYCIN INCREASES LENGTH AND MECHANOSENSORY FUNCTION OF PRIMARY CILIA IN RENAL EPITHELIAL AND VASCULAR ENDOTHELIAL CELLS.Int Educ Res J. 2016 Dec;2(12):91-97. Int Educ Res J. 2016. PMID: 28529994 Free PMC article.
-
Renal cilia display length alterations following tubular injury and are present early in epithelial repair.Nephrol Dial Transplant. 2008 Mar;23(3):834-41. doi: 10.1093/ndt/gfm743. Epub 2007 Oct 25. Nephrol Dial Transplant. 2008. PMID: 17962379
Cited by
-
Primary cilia and lipid raft dynamics.Open Biol. 2021 Aug;11(8):210130. doi: 10.1098/rsob.210130. Epub 2021 Aug 25. Open Biol. 2021. PMID: 34428960 Free PMC article. Review.
-
Primary Cilia: A Cellular Regulator of Articular Cartilage Degeneration.Stem Cells Int. 2022 Sep 23;2022:2560441. doi: 10.1155/2022/2560441. eCollection 2022. Stem Cells Int. 2022. PMID: 36193252 Free PMC article. Review.
-
A loss of primary cilia by a reduction in mTOR signaling correlates with age-related deteriorations in condylar cartilage.Geroscience. 2024 Dec;46(6):5995-6007. doi: 10.1007/s11357-024-01143-x. Epub 2024 Mar 25. Geroscience. 2024. PMID: 38526843 Free PMC article.
-
Subtype-Selective Positive Modulation of KCa2.3 Channels Increases Cilia Length.ACS Chem Biol. 2022 Aug 19;17(8):2344-2354. doi: 10.1021/acschembio.2c00469. Epub 2022 Aug 10. ACS Chem Biol. 2022. PMID: 35947779 Free PMC article.
-
Folate conjugated nanomedicines for selective inhibition of mTOR signaling in polycystic kidneys at clinically relevant doses.Biomaterials. 2023 Nov;302:122329. doi: 10.1016/j.biomaterials.2023.122329. Epub 2023 Sep 13. Biomaterials. 2023. PMID: 37722182 Free PMC article.
References
-
- Schneider L, Cammer M, Lehman J, Nielsen SK, Guerra CF, Veland IR,Stock C, Hoffmann EK, Yoder BK, Schwab A, Satir P, Christensen ST, Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts, Cell Physiol. Biochem 25 (2–3) (2010) 279–292. - PMC - PubMed

