High-Throughput Screening at the Membrane Interface Reveals Inhibitors of Amyloid-β
- PMID: 32469202
- PMCID: PMC10323872
- DOI: 10.1021/acs.biochem.0c00328
High-Throughput Screening at the Membrane Interface Reveals Inhibitors of Amyloid-β
Abstract
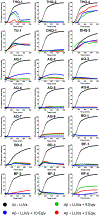
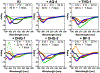
Aggregation and the formation of oligomeric intermediates of amyloid-β (Aβ) at the membrane interface of neuronal cells are implicated in the cellular toxicity and pathology of Alzheimer's disease. Small molecule compounds have been shown to suppress amyloid aggregation and cellular toxicity, but often the presence of a lipid membrane negates their activity. A high-throughput screen of 1800 small molecules was performed to search for membrane active inhibitors, and 21 primary hits were discovered. Through the use of fluorescence-based assays, transmission electron microscopy, and dot blot assays, the initial 21 primary hits were narrowed down to five lead compounds. Nuclear magnetic resonance and circular dichroism experiments were used for further confirmation of amyloid inhibition at the membrane interface and to obtain insights into the secondary structure of amyloid-β, while size exclusion chromatography was used to characterize the size of Aβ species. Lastly, dye-leakage assays allowed us to understand how the addition of the five lead compounds affected amyloid-β's ability to permeate the lipid bilayer. These results provide insights into small molecules that stabilize small amyloid species in the presence of membranes for the development of tool compounds for deeper investigations of these transient species.
Figures

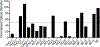

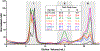
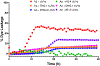
Similar articles
-
Reduced Lipid Bilayer Thickness Regulates the Aggregation and Cytotoxicity of Amyloid-β.J Biol Chem. 2017 Mar 17;292(11):4638-4650. doi: 10.1074/jbc.M116.764092. Epub 2017 Feb 1. J Biol Chem. 2017. PMID: 28154182 Free PMC article.
-
Structural evolution and membrane interaction of the 40-residue β amyloid peptides: differences in the initial proximity between peptides and the membrane bilayer studied by solid-state nuclear magnetic resonance spectroscopy.Biochemistry. 2014 Dec 9;53(48):7503-14. doi: 10.1021/bi501003n. Epub 2014 Nov 26. Biochemistry. 2014. PMID: 25397729
-
Amyloid-β oligomers have a profound detergent-like effect on lipid membrane bilayers, imaged by atomic force and electron microscopy.J Biol Chem. 2019 May 10;294(19):7566-7572. doi: 10.1074/jbc.AC118.007195. Epub 2019 Apr 3. J Biol Chem. 2019. PMID: 30948512 Free PMC article.
-
Amyloid-β Interactions with Lipid Rafts in Biomimetic Systems: A Review of Laboratory Methods.Methods Mol Biol. 2021;2187:47-86. doi: 10.1007/978-1-0716-0814-2_4. Methods Mol Biol. 2021. PMID: 32770501 Review.
-
Solid-state NMR as a method to reveal structure and membrane-interaction of amyloidogenic proteins and peptides.Biochim Biophys Acta. 2007 Aug;1768(8):1900-12. doi: 10.1016/j.bbamem.2007.03.025. Epub 2007 Apr 5. Biochim Biophys Acta. 2007. PMID: 17524351 Review.
Cited by
-
Metal-Complexes Bearing Releasable CO Differently Modulate Amyloid Aggregation.Inorg Chem. 2023 Jul 3;62(26):10470-10480. doi: 10.1021/acs.inorgchem.3c01522. Epub 2023 Jun 20. Inorg Chem. 2023. PMID: 37338927 Free PMC article.
-
Alleviating the unwanted effects of oxidative stress on Aβ clearance: a review of related concepts and strategies for the development of computational modelling.Transl Neurodegener. 2023 Mar 13;12(1):11. doi: 10.1186/s40035-023-00344-2. Transl Neurodegener. 2023. PMID: 36907887 Free PMC article. Review.
-
Novel Peptide-Calix[4]arene Conjugate Inhibits Aβ Aggregation and Rescues Neurons from Aβ's Oligomers Cytotoxicity In Vitro.ACS Chem Neurosci. 2021 Apr 21;12(8):1449-1462. doi: 10.1021/acschemneuro.1c00117. Epub 2021 Apr 12. ACS Chem Neurosci. 2021. PMID: 33844495 Free PMC article.
-
Gallium nanoparticles as novel inhibitors of Aβ40 aggregation.Mater Adv. 2021 Jul 9;2(16):5471-5478. doi: 10.1039/d1ma00461a. eCollection 2021 Aug 16. Mater Adv. 2021. PMID: 34458846 Free PMC article.
-
Amyloid Oligomers: A Joint Experimental/Computational Perspective on Alzheimer's Disease, Parkinson's Disease, Type II Diabetes, and Amyotrophic Lateral Sclerosis.Chem Rev. 2021 Feb 24;121(4):2545-2647. doi: 10.1021/acs.chemrev.0c01122. Epub 2021 Feb 5. Chem Rev. 2021. PMID: 33543942 Free PMC article. Review.
References
-
- Hamley IW The Amyloid Beta Peptide: A Chemist’s Perspective. Role in Alzheimer’s and Fibrillization. Chem. Rev 2012, 112 (10), 5147–5192. - PubMed
-
- Benilova I; Karran E; De Strooper B The Toxic Aβ Oligomer and Alzheimer’s Disease: An Emperor in Need of Clothes. Nat. Neurosci 2012, 15 (3), 349–357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

