Risk of early progression according to circulating ESR1 mutation, CA-15.3 and cfDNA increases under first-line anti-aromatase treatment in metastatic breast cancer
- PMID: 32466779
- PMCID: PMC7254698
- DOI: 10.1186/s13058-020-01290-x
Risk of early progression according to circulating ESR1 mutation, CA-15.3 and cfDNA increases under first-line anti-aromatase treatment in metastatic breast cancer
Abstract
Background: Endocrine therapy is recommended as a first-line treatment for hormone receptor-positive metastatic breast cancer (HR+MBC) patients. No biomarker has been validated to predict tumor progression in that setting. We aimed to prospectively compare the risk of early progression according to circulating ESR1 mutations, CA-15.3, and circulating cell-free DNA in MBC patients treated with a first-line aromatase inhibitor (AI).
Methods: Patients with MBC treated with a first-line AI were prospectively included. Circulating biomarker assessment was performed every 3 months. The primary objective was to determine the risk of progression or death at the next follow-up visit (after 3 months) in case of circulating ESR1 mutation detection among patients treated with a first-line AI for HR+MBC.
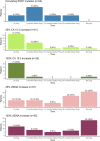
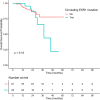
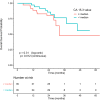
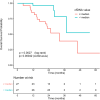
Results: Overall, 103 patients were included, and 70 (68%) had progressive disease (PD). Circulating ESR1 mutations were detected in 22/70 patients with PD and in 0/33 patients without progression (p < 0.001). Among the ESR1-mutated patients, 18/22 had a detectable mutation prior to progression, with a median delay of 110 days from first detection to PD. The detection of circulating ESR1 mutations was associated with a 4.9-fold (95% CI 3.0-8.0) increase in the risk of PD at 3 months. Using a threshold value of 25% or 100%, a CA-15.3 increase was also correlated with progression (p < 0.001 and p = 0.003, respectively). In contrast to ESR1, the CA-15.3 increase occurred concomitantly with PD in most cases, in 27/47 (57%) with a 25% threshold and in 21/25 (84%) with a 100% threshold. Using a threshold value of either 25% or 100%, cfDNA increase was not correlated with progression.
Conclusion: The emergence of circulating ESR1 mutations is associated with a 4.9-fold increase in the risk of early PD during AI treatment in HR+MBC. Our results also highlighted that tracking circulating ESR1 mutations is more relevant than tracking CA-15.3 or cfDNA increase to predict progression in this setting.
Trial registration: ClinicalTrials.gov, NCT02473120. Registered 16 June 2015-retrospectively registered after one inclusion (first inclusion 1 June 2015).
Keywords: Aromatase inhibitor; Breast cancer; CA-15.3; Cell-free DNA; Circulating DNA; ESR1 mutation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Tracking evolution of aromatase inhibitor resistance with circulating tumour DNA analysis in metastatic breast cancer.Ann Oncol. 2018 Jan 1;29(1):145-153. doi: 10.1093/annonc/mdx483. Ann Oncol. 2018. PMID: 29045530 Free PMC article.
-
Clinical significance of monitoring ESR1 mutations in circulating cell-free DNA in estrogen receptor positive breast cancer patients.Oncotarget. 2016 May 31;7(22):32504-18. doi: 10.18632/oncotarget.8839. Oncotarget. 2016. PMID: 27102299 Free PMC article.
-
Kinetics, prognostic and predictive values of ESR1 circulating mutations in metastatic breast cancer patients progressing on aromatase inhibitor.Oncotarget. 2016 Nov 15;7(46):74448-74459. doi: 10.18632/oncotarget.12950. Oncotarget. 2016. PMID: 27801670 Free PMC article.
-
The association between type of endocrine therapy and development of estrogen receptor-1 mutation(s) in patients with hormone-sensitive advanced breast cancer: A systematic review and meta-analysis of randomized and non-randomized trials.Biochim Biophys Acta Rev Cancer. 2019 Dec;1872(2):188315. doi: 10.1016/j.bbcan.2019.188315. Epub 2019 Oct 21. Biochim Biophys Acta Rev Cancer. 2019. PMID: 31647985 Review.
-
[Clinical relevance of ESR1 circulating mutations detection in hormone receptor positive metastatic breast cancer].Bull Cancer. 2018 Jan;105(1):46-54. doi: 10.1016/j.bulcan.2017.09.002. Epub 2017 Oct 9. Bull Cancer. 2018. PMID: 29032804 Review. French.
Cited by
-
Identification of molecular biomarkers and pathways of NSCLC: insights from a systems biomedicine perspective.J Genet Eng Biotechnol. 2021 Mar 19;19(1):43. doi: 10.1186/s43141-021-00134-1. J Genet Eng Biotechnol. 2021. PMID: 33742334 Free PMC article.
-
Cell-Free DNA Variables including Gene Mutations in CA15-3 Normal Breast Cancer Reflect Prognosis.Dis Markers. 2022 Feb 24;2022:5470166. doi: 10.1155/2022/5470166. eCollection 2022. Dis Markers. 2022. PMID: 35251373 Free PMC article.
-
Metastatic ER+ Breast Cancer: Mechanisms of Resistance and Future Therapeutic Approaches.Int J Mol Sci. 2023 Nov 11;24(22):16198. doi: 10.3390/ijms242216198. Int J Mol Sci. 2023. PMID: 38003387 Free PMC article. Review.
-
Circulating tumor DNA predicts efficacy of a dual AKT/p70S6K inhibitor (LY2780301) plus paclitaxel in metastatic breast cancer: plasma analysis of the TAKTIC phase IB/II study.Mol Oncol. 2022 May;16(10):2057-2070. doi: 10.1002/1878-0261.13188. Epub 2022 Mar 30. Mol Oncol. 2022. PMID: 35122700 Free PMC article. Clinical Trial.
-
A Systematic Review of the Use of Circulating Cell-Free DNA Dynamics to Monitor Response to Treatment in Metastatic Breast Cancer Patients.Cancers (Basel). 2021 Apr 10;13(8):1811. doi: 10.3390/cancers13081811. Cancers (Basel). 2021. PMID: 33920135 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

