Design of a surrogate Anticalin protein directed against CD98hc for preclinical studies in mice
- PMID: 32463547
- PMCID: PMC7380666
- DOI: 10.1002/pro.3894
Design of a surrogate Anticalin protein directed against CD98hc for preclinical studies in mice
Abstract
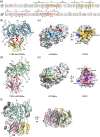
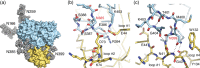
The human CD98 heavy chain (CD98hc) offers a promising biomedical target both for tumor therapy and for drug delivery to the brain. We have previously developed a cognate Anticalin protein with picomolar affinity and demonstrated its effectiveness in a xenograft animal model. Due to the lack of cross-reactivity with the murine ortholog, we now report the development and X-ray structural analysis of an Anticalin with high affinity toward CD98hc from mouse. This binding protein recognizes the same protruding epitope loop-despite distinct structure-in the membrane receptor ectodomain as the Anticalin selected against human CD98hc. Thus, this surrogate Anticalin should be useful for the preclinical assessment of CD98hc targeting in vivo and support the translational development for medical application in humans.
Keywords: CD98hc; cancer theranostics; lipocalin; mouse model; protein engineering.
© 2020 The Authors. Protein Science published by Wiley Periodicals, Inc. on behalf of The Protein Society.
Conflict of interest statement
A. Skerra is founder and shareholder of Pieris Pharmaceuticals, Inc.
Figures

Similar articles
-
Development of a high affinity Anticalin® directed against human CD98hc for theranostic applications.Theranostics. 2020 Jan 12;10(5):2172-2187. doi: 10.7150/thno.38968. eCollection 2020. Theranostics. 2020. PMID: 32089738 Free PMC article.
-
Structural differences between the ectodomains of murine and human CD98hc.Proteins. 2019 Aug;87(8):693-698. doi: 10.1002/prot.25686. Epub 2019 Apr 23. Proteins. 2019. PMID: 30958588
-
The IMiD target CRBN determines HSP90 activity toward transmembrane proteins essential in multiple myeloma.Mol Cell. 2021 Mar 18;81(6):1170-1186.e10. doi: 10.1016/j.molcel.2020.12.046. Epub 2021 Feb 10. Mol Cell. 2021. PMID: 33571422 Free PMC article.
-
The role of CD98 heavy chain in cancer development.Histol Histopathol. 2024 Dec;39(12):1557-1564. doi: 10.14670/HH-18-749. Epub 2024 Apr 16. Histol Histopathol. 2024. PMID: 38695393 Review.
-
CD98hc in host-pathogen interactions: roles of the multifunctional host protein during infections.FEMS Microbiol Rev. 2022 Sep 2;46(5):fuac023. doi: 10.1093/femsre/fuac023. FEMS Microbiol Rev. 2022. PMID: 35595511 Review.
References
-
- Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J Biol Chem. 1998;273:23629–23632. - PubMed
-
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem. 1999;274:11455–11458. - PubMed
-
- Fotiadis D, Kanai Y, Palacin M. The SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med. 2013;34:139–158. - PubMed
-
- Nakamura E, Sato M, Yang H, et al. 4F2 (CD98) heavy chain is associated covalently with an amino acid transporter and controls intracellular trafficking and membrane topology of 4F2 heterodimer. J Biol Chem. 1999;274:3009–3016. - PubMed
-
- Yan R, Zhao X, Lei J, Zhou Q. Structure of the human LAT1‐4F2hc heteromeric amino acid transporter complex. Nature. 2019;568:127–130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

