Contribution of NKT cells to the immune response and pathogenesis triggered by respiratory viruses
- PMID: 32463330
- PMCID: PMC7549913
- DOI: 10.1080/21505594.2020.1770492
Contribution of NKT cells to the immune response and pathogenesis triggered by respiratory viruses
Abstract
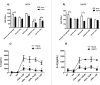
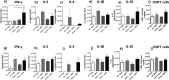
Human respiratory syncytial virus (hRSV) and human metapneumovirus (hMPV) cause acute respiratory tract infections in children worldwide. Natural killer T (NKT) cells are unconventional T lymphocytes, and their TCRs recognize glycolipids bound to the MHC-I-like molecule, CD1d. These cells modulate the inflammatory response in viral infections. Here, we evaluated the contribution of NKT cells in both hRSV and hMPV infections. A significant decrease in the number of neutrophils, eosinophils, and CD103+DCs infiltrating to the lungs, as well as an increased production of IFN-γ, were observed upon hRSV-infection in CD1d-deficient BALB/c mice, as compared to wild-type control mice. However, this effect was not observed in the CD1d-deficient BALB/c group, upon infection with hMPV. Importantly, reduced expression of CD1d in CD11b+ DCs and epithelial cells was found in hRSV -but not hMPV-infected mice. Besides, a reduction in the expression of CD1d in alveolar macrophages of lungs from hRSV- and hMPV-infected mice was found. Such reduction of CD1d expression interfered with NKT cells activation, and consequently IL-2 secretion, as characterized by in vitro experiments for both hRSV and hMPV infections. Furthermore, increased numbers of NKT cells recruited to the lungs in response to hRSV- but not hMPV-infection was detected, resulting in a reduction in the expression of IFN-γ and IL-2 by these cells. In conclusion, both hRSV and hMPV might be differently impairing NKT cells function and contributing to the immune response triggered by these viruses.
Keywords: Human respiratory syncytial virus; human metapneumovirus; natural killer T cells; pulmonary inflammation; viral infection.
Conflict of interest statement
SAP is a paid consultant for Vaccinex (Rochester, NY, USA), which is a privately held biotechnology company with preclinical programs for the development of NKT cells-based vaccines and immunotherapies. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Alveolar macrophages contribute to the pathogenesis of human metapneumovirus infection while protecting against respiratory syncytial virus infection.Am J Respir Cell Mol Biol. 2014 Oct;51(4):502-15. doi: 10.1165/rcmb.2013-0414OC. Am J Respir Cell Mol Biol. 2014. PMID: 24749674 Free PMC article.
-
Human metapneumovirus induces more severe disease and stronger innate immune response in BALB/c mice as compared with respiratory syncytial virus.Respir Res. 2007 Jan 29;8(1):6. doi: 10.1186/1465-9921-8-6. Respir Res. 2007. PMID: 17257445 Free PMC article.
-
Aberrant T cell immunity triggered by human Respiratory Syncytial Virus and human Metapneumovirus infection.Virulence. 2017 Aug 18;8(6):685-704. doi: 10.1080/21505594.2016.1265725. Epub 2016 Dec 2. Virulence. 2017. PMID: 27911218 Free PMC article. Review.
-
Innate Immune Components that Regulate the Pathogenesis and Resolution of hRSV and hMPV Infections.Viruses. 2020 Jun 12;12(6):637. doi: 10.3390/v12060637. Viruses. 2020. PMID: 32545470 Free PMC article. Review.
-
Pulmonary infection of mice with human metapneumovirus induces local cytotoxic T-cell and immunoregulatory cytokine responses similar to those seen with human respiratory syncytial virus.J Gen Virol. 2010 May;91(Pt 5):1302-10. doi: 10.1099/vir.0.015396-0. Epub 2010 Jan 6. J Gen Virol. 2010. PMID: 20053825
Cited by
-
Die Kämpfe únd schláchten-the struggles and battles of innate-like effector T lymphocytes with microbes.Front Immunol. 2023 Apr 24;14:1117825. doi: 10.3389/fimmu.2023.1117825. eCollection 2023. Front Immunol. 2023. PMID: 37168859 Free PMC article. Review.
-
Type I Natural Killer T Cells as Key Regulators of the Immune Response to Infectious Diseases.Clin Microbiol Rev. 2020 Dec 23;34(2):e00232-20. doi: 10.1128/CMR.00232-20. Print 2021 Mar 17. Clin Microbiol Rev. 2020. PMID: 33361143 Free PMC article. Review.
-
Glycolipid antigen recognition by invariant natural killer T cells and its role in homeostasis and antimicrobial responses.Front Immunol. 2024 May 28;15:1402412. doi: 10.3389/fimmu.2024.1402412. eCollection 2024. Front Immunol. 2024. PMID: 38863694 Free PMC article. Review.
-
Single-Cell Antigen Receptor Sequencing in Pigs with Influenza.bioRxiv [Preprint]. 2024 Oct 15:2024.10.13.617920. doi: 10.1101/2024.10.13.617920. bioRxiv. 2024. PMID: 39464079 Free PMC article. Preprint.
-
Natural Killer T Cell Diversity and Immunotherapy.Cancers (Basel). 2023 Dec 7;15(24):5737. doi: 10.3390/cancers15245737. Cancers (Basel). 2023. PMID: 38136283 Free PMC article. Review.
References
-
- Liu J, Wu J, Qi F, et al. Natural helper cells contribute to pulmonary eosinophilia by producing IL-13 via IL-33/ST2 pathway in a murine model of respiratory syncytial virus infection. Int Immunopharmacol. 2015;28(1):337–343. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
