Mobile Delivery of the Diabetes Prevention Program in People With Prediabetes: Randomized Controlled Trial
- PMID: 32459631
- PMCID: PMC7381044
- DOI: 10.2196/17842
Mobile Delivery of the Diabetes Prevention Program in People With Prediabetes: Randomized Controlled Trial
Abstract
Background: The Centers for Disease Control and Prevention (CDC) diabetes prevention program (DPP) has formed the foundation for Type 2 Diabetes Mellitus (T2DM) prevention efforts and lifestyle change modifications in multiple care settings. To our knowledge, no randomized controlled trial has verified the efficacy of a fully mobile version of CDC's diabetes prevention program (DPP).
Objective: This study aimed to investigate the long-term weight loss and glycemic efficacy of a mobile-delivered DPP compared with a control group receiving usual medical care.
Methods: Adults with prediabetes (N=202) were recruited from a clinic and randomized to either a mobile-delivered, coach-guided DPP (Noom) or a control group that received regular medical care including a paper-based DPP curriculum and no formal intervention. The intervention group learned how to use the Noom program, how to interact with their coach, and the importance of maintaining motivation. They had access to an interactive coach-to-participant interface and group messaging, daily challenges for behavior change, DPP-based education articles, food logging, and automated feedback. Primary outcomes included changes in weight and hemoglobin A1c (HbA1c) levels at 6 and 12 months, respectively. Exploratory secondary outcomes included program engagement as a predictor of changes in weight and HbA1c levels.
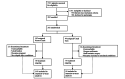
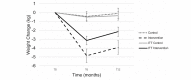
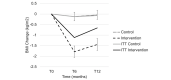
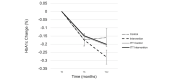
Results: A total of 202 participants were recruited and randomized into the intervention (n=101) or control group (n=99). In the intention-to-treat (ITT) analyses, changes in the participants' weight and BMI were significantly different at 6 months between the intervention and control groups, but there was no difference in HbA1c levels (mean difference 0.004%, SE 0.05; P=.94). Weight and BMI were lower in the intervention group by -2.64 kg (SE 0.71; P<.001) and -0.99 kg/m2 (SE 0.29; P=.001), respectively. These differences persisted at 12 months. However, in the analyses that did not involve ITT, program completers achieved a significant weight loss of 5.6% (SE 0.81; P<.001) at 6 months, maintaining 4.7% (SE 0.88; P<.001) of their weight loss at 12 months. The control group lost -0.15% at 6 months (SE 0.64; P=.85) and gained 0.33% (SE 0.70; P=.63) at 12 months. Those randomized to the intervention group who did not start the program had no meaningful weight or HbA1c level change, similar to the control group. At 1 year, the intervention group showed a 0.23% reduction in HbA1c levels; those who completed the intervention showed a 0.28% reduction. Those assigned to the control group had a 0.16% reduction in HbA1c levels.
Conclusions: This novel mobile-delivered DPP achieved significant weight loss reductions for up to 1 year compared with usual care. This type of intervention reduces the risk of overt diabetes without the added barriers of in-person interventions.
Trial registration: ClinicalTrials.gov NCT03865342; https://clinicaltrials.gov/ct2/show/NCT03865342.
Keywords: body weight; mHealth; mobile app; mobile phone; prediabetes; randomized controlled trial.
©Tatiana Toro-Ramos, Andreas Michaelides, Maria Anton, Zulekha Karim, Leah Kang-Oh, Charalambos Argyrou, Elisavet Loukaidou, Marina M Charitou, Wilson Sze, Joshua D Miller. Originally published in JMIR mHealth and uHealth (http://mhealth.jmir.org), 08.07.2020.
Conflict of interest statement
Conflicts of Interest: TT and AM are employed by Noom, Inc and receive salary and stock options. AM holds a patent pending (3492.004US1) with Noom, Inc. Noom Coach is a mHealth program owned by Noom, Inc. The authors have no additional conflicts of interest, and no competing financial interests exist for the other authors.
Figures
Similar articles
-
Diabetes Prevention and Weight Loss with a Fully Automated Behavioral Intervention by Email, Web, and Mobile Phone: A Randomized Controlled Trial Among Persons with Prediabetes.J Med Internet Res. 2015 Oct 23;17(10):e240. doi: 10.2196/jmir.4897. J Med Internet Res. 2015. PMID: 26499966 Free PMC article. Clinical Trial.
-
Effectiveness of artificial intelligence vs. human coaching in diabetes prevention: a study protocol for a randomized controlled trial.Trials. 2024 May 16;25(1):325. doi: 10.1186/s13063-024-08177-8. Trials. 2024. PMID: 38755706 Free PMC article.
-
A Mobile Phone-Based Program to Promote Healthy Behaviors Among Adults With Prediabetes Who Declined Participation in Free Diabetes Prevention Programs: Mixed-Methods Pilot Randomized Controlled Trial.JMIR Mhealth Uhealth. 2019 Jan 9;7(1):e11267. doi: 10.2196/11267. JMIR Mhealth Uhealth. 2019. PMID: 30626566 Free PMC article. Clinical Trial.
-
Behavioral and Pharmacotherapy Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: An Updated Systematic Review for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Sep. Report No.: 18-05239-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Sep. Report No.: 18-05239-EF-1. PMID: 30354042 Free Books & Documents. Review.
-
Cardiometabolic Risk Factor Changes Observed in Diabetes Prevention Programs in US Settings: A Systematic Review and Meta-analysis.PLoS Med. 2016 Jul 26;13(7):e1002095. doi: 10.1371/journal.pmed.1002095. eCollection 2016 Jul. PLoS Med. 2016. PMID: 27459705 Free PMC article. Review.
Cited by
-
Feasibility of an Interactive Health Coaching Mobile App to Prevent Malnutrition and Muscle Loss in Esophageal Cancer Patients Receiving Neoadjuvant Concurrent Chemoradiotherapy: Prospective Pilot Study.J Med Internet Res. 2021 Aug 27;23(8):e28695. doi: 10.2196/28695. J Med Internet Res. 2021. PMID: 34448714 Free PMC article.
-
Efficacy of a Multimodal Digital Behavior Change Intervention on Lifestyle Behavior, Cardiometabolic Biomarkers, and Medical Expenditure: Protocol for a Randomized Controlled Trial.JMIR Res Protoc. 2024 Oct 30;13:e50378. doi: 10.2196/50378. JMIR Res Protoc. 2024. PMID: 39475852 Free PMC article. Clinical Trial.
-
A Pilot Study to Examine the Feasibility and Acceptability of a Virtual Adaptation of an In-Person Adolescent Diabetes Prevention Program.Int J Environ Res Public Health. 2022 Sep 27;19(19):12286. doi: 10.3390/ijerph191912286. Int J Environ Res Public Health. 2022. PMID: 36231588 Free PMC article.
-
Analyzing the Effectiveness of mHealth to Manage Diabetes Mellitus Among Adults Over 50: A Systematic Literature Review.J Multidiscip Healthc. 2023 Jan 12;16:101-117. doi: 10.2147/JMDH.S392693. eCollection 2023. J Multidiscip Healthc. 2023. PMID: 36660039 Free PMC article. Review.
-
Weight loss in a digital app-based diabetes prevention program powered by artificial intelligence.Digit Health. 2022 Oct 9;8:20552076221130619. doi: 10.1177/20552076221130619. eCollection 2022 Jan-Dec. Digit Health. 2022. PMID: 36238752 Free PMC article.
References
-
- CDC Centers for Disease Control and Prevention. 2017. [2020-05-07]. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States https://www.cdc.gov/diabetes/data/statistics/statistics-report.html.
-
- World Health Organization. 2016. [2019-03-28]. Global Report on Diabetes https://www.who.int/diabetes/global-report/en/:
-
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010 Oct 22;8:29. doi: 10.1186/1478-7954-8-29. https://pophealthmetrics.biomedcentral.com/articles/10.1186/1478-7954-8-29 - DOI - DOI - PMC - PubMed
-
- American Diabetes Association Economic costs of diabetes in the US In 2017. Diabetes Care. 2018 May;41(5):917–28. doi: 10.2337/dci18-0007. http://europepmc.org/abstract/MED/29567642 - DOI - PMC - PubMed
-
- Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008 Apr 1;26(2):77–82. doi: 10.2337/diaclin.26.2.77. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

