Nanotechnology-Based Vaccines for Allergen-Specific Immunotherapy: Potentials and Challenges of Conventional and Novel Adjuvants under Research
- PMID: 32443671
- PMCID: PMC7349961
- DOI: 10.3390/vaccines8020237
Nanotechnology-Based Vaccines for Allergen-Specific Immunotherapy: Potentials and Challenges of Conventional and Novel Adjuvants under Research
Abstract
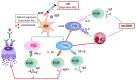
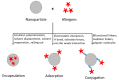
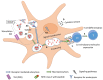
The increasing prevalence of allergic diseases demands efficient therapeutic strategies for their mitigation. Allergen-specific immunotherapy (AIT) is the only causal rather than symptomatic treatment method available for allergy. Currently, AIT is being administered using immune response modifiers or adjuvants. Adjuvants aid in the induction of a vigorous and long-lasting immune response, thereby improving the efficiency of AIT. The successful development of a novel adjuvant requires a thorough understanding of the conventional and novel adjuvants under development. Thus, this review discusses the potentials and challenges of these adjuvants and their mechanism of action. Vaccine development based on nanoparticles is a promising strategy for AIT, due to their inherent physicochemical properties, along with their ease of production and ability to stimulate innate immunity. Although nanoparticles have provided promising results as an adjuvant for AIT in in vivo studies, a deeper insight into the interaction of nanoparticle-allergen complexes with the immune system is necessary. This review focuses on the methods of harnessing the adjuvant effect of nanoparticles by detailing the molecular mechanisms underlying the immune response, which includes allergen uptake, processing, presentation, and induction of T cell differentiation.
Keywords: AIT; CpG oligonucleotide; allergy; alum; calcium phosphate; microcrystalline tyrosine; nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest with regard to the content of this review.
Figures

Similar articles
-
Allergens and Adjuvants in Allergen Immunotherapy for Immune Activation, Tolerance, and Resilience.J Allergy Clin Immunol Pract. 2021 May;9(5):1780-1789. doi: 10.1016/j.jaip.2020.12.008. Epub 2021 Mar 19. J Allergy Clin Immunol Pract. 2021. PMID: 33753052
-
Clinical use of adjuvants in allergen-immunotherapy.Expert Rev Clin Immunol. 2017 Jun;13(6):599-610. doi: 10.1080/1744666X.2017.1292133. Epub 2017 Feb 23. Expert Rev Clin Immunol. 2017. PMID: 28162007 Review.
-
Novel adjuvants in allergen-specific immunotherapy: where do we stand?Front Immunol. 2024 Feb 23;15:1348305. doi: 10.3389/fimmu.2024.1348305. eCollection 2024. Front Immunol. 2024. PMID: 38464539 Free PMC article. Review.
-
Microcrystalline Tyrosine and Aluminum as Adjuvants in Allergen-Specific Immunotherapy Protect from IgE-Mediated Reactivity in Mouse Models and Act Independently of Inflammasome and TLR Signaling.J Immunol. 2018 May 1;200(9):3151-3159. doi: 10.4049/jimmunol.1800035. Epub 2018 Mar 28. J Immunol. 2018. PMID: 29592962 Free PMC article.
-
Adjuvants in Allergen-Specific Immunotherapy: Modulating and Enhancing the Immune Response.J Investig Allergol Clin Immunol. 2019;29(2):103-111. doi: 10.18176/jiaci.0349. Epub 2018 Nov 12. J Investig Allergol Clin Immunol. 2019. PMID: 30418155 Review.
Cited by
-
Suitability of potyviral recombinant virus-like particles bearing a complete food allergen for immunotherapy vaccines.Front Immunol. 2022 Sep 8;13:986823. doi: 10.3389/fimmu.2022.986823. eCollection 2022. Front Immunol. 2022. PMID: 36159839 Free PMC article.
-
Allergic Rhinitis: What Do We Know About Allergen-Specific Immunotherapy?Front Allergy. 2021 Oct 28;2:747323. doi: 10.3389/falgy.2021.747323. eCollection 2021. Front Allergy. 2021. PMID: 35387059 Free PMC article. Review.
-
Emerging strategies for combating Fusobacterium nucleatum in colorectal cancer treatment: Systematic review, improvements and future challenges.Exploration (Beijing). 2023 Dec 22;4(1):20230092. doi: 10.1002/EXP.20230092. eCollection 2024 Feb. Exploration (Beijing). 2023. PMID: 38854496 Free PMC article. Review.
-
Engineering customized nanovaccines for enhanced cancer immunotherapy.Bioact Mater. 2024 Mar 10;36:330-357. doi: 10.1016/j.bioactmat.2024.02.028. eCollection 2024 Jun. Bioact Mater. 2024. PMID: 38496036 Free PMC article. Review.
-
Nanovaccines against Viral Infectious Diseases.Pharmaceutics. 2022 Nov 22;14(12):2554. doi: 10.3390/pharmaceutics14122554. Pharmaceutics. 2022. PMID: 36559049 Free PMC article. Review.
References
-
- Muraro A. EAACI Advocacy Manifesto, Tackling the Allergy Crisis in Europe-Concerted Policy Action Needed. [(accessed on 20 November 2018)]; Available online: https://www.eaaci.org/documents/EAACI_Advocacy_Manifesto.pdf.
-
- Benton E.N., Sayes C.M.J.J.E.S. Environmental Factors Contribute to the Onset of Food Allergies. J. Environ. Sci. 2017;1:27–43. doi: 10.26502/jesph.9612003. - DOI
-
- Himly M., Grotz B., Sageder M., Geppert M., Duschl A.J.C.B. Immune frailty and nanomaterials: The case of allergies. Curr. Bionanotechnol. 2016;2:20–28. doi: 10.2174/2213529402666160601124654. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

