Pollen Proteases Play Multiple Roles in Allergic Disorders
- PMID: 32438574
- PMCID: PMC7278992
- DOI: 10.3390/ijms21103578
Pollen Proteases Play Multiple Roles in Allergic Disorders
Abstract
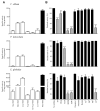
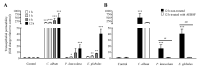
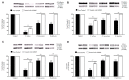
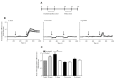
Allergic diseases are a major health concern worldwide. Pollens are important triggers for allergic rhinitis, conjunctivitis and asthma. Proteases released upon pollen grain hydration appear to play a major role in the typical immunological and inflammatory responses that occur in patients with allergic disorders. In this study, we aimed to identify specific proteolytic activity in a set of pollens with diverse allergenic potential. Diffusates from Chenopodium album, Plantago lanceolata and Eucalyptus globulus were added to a confluent monolayer of Calu-3 cells grown in an air-liquid interface system. We identified serine proteases and metalloproteinases in all pollen diffusates investigated. Proteases found in these pollen diffusates were shown to compromise the integrity of the lung epithelial barrier by disrupting transmembrane adhesion proteins E-cadherin, claudin-1 and Occludin, as well as, the cytosolic complex zonula occludens-1 (ZO-1) resulting in a time-dependent increase in transepithelial permeability. Tight junction disruption and increased transepithelial permeability facilitates allergen exposure to epithelial sub-layers contributing to the sensitization to a wide range of allergens. These pollen extracts also induced an increase in the release of interleukin 6 (IL-6) and interleukin 8 (IL-8) cytokines measured by flow cytometry possibly as a result of the activation of protease-activated receptors 2 (PAR-2).
Keywords: IL-6; IL-8 and PAR-2; allergy; pollen proteases; transepithelial permeability.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Pollen proteases compromise the airway epithelial barrier through degradation of transmembrane adhesion proteins and lung bioactive peptides.Allergy. 2011 Aug;66(8):1088-98. doi: 10.1111/j.1398-9995.2011.02598.x. Epub 2011 Apr 11. Allergy. 2011. PMID: 21480927
-
Pollen proteolytic enzymes degrade tight junctions.Respirology. 2007 Nov;12(6):834-42. doi: 10.1111/j.1440-1843.2007.01175.x. Respirology. 2007. PMID: 17986111
-
Mass spectrometric analysis of electrophoretically separated allergens and proteases in grass pollen diffusates.Respir Res. 2003;4(1):10. doi: 10.1186/1465-9921-4-10. Epub 2003 Sep 20. Respir Res. 2003. PMID: 14577842 Free PMC article.
-
The role of protease activation of inflammation in allergic respiratory diseases.J Allergy Clin Immunol. 2004 Nov;114(5):997-1008; quiz 1009. doi: 10.1016/j.jaci.2004.07.060. J Allergy Clin Immunol. 2004. PMID: 15536399 Review.
-
The pollen enigma: modulation of the allergic immune response by non-allergenic, pollen-derived compounds.Curr Pharm Des. 2012;18(16):2314-9. doi: 10.2174/138161212800166040. Curr Pharm Des. 2012. PMID: 22390694 Review.
Cited by
-
Ozone impairs endogenous compensatory responses in allergic asthma.Toxicol Appl Pharmacol. 2023 Jan 15;459:116341. doi: 10.1016/j.taap.2022.116341. Epub 2022 Dec 8. Toxicol Appl Pharmacol. 2023. PMID: 36502870 Free PMC article.
-
Effects of a Cloth Panel Containing a Specific Ore Powder on Patients with Chamaecyparis obtusa (Cypress) Pollen Allergy.ScientificWorldJournal. 2021 Nov 10;2021:3924393. doi: 10.1155/2021/3924393. eCollection 2021. ScientificWorldJournal. 2021. PMID: 34803524 Free PMC article. Clinical Trial.
-
The Dual Role of the Airway Epithelium in Asthma: Active Barrier and Regulator of Inflammation.Cells. 2023 Sep 5;12(18):2208. doi: 10.3390/cells12182208. Cells. 2023. PMID: 37759430 Free PMC article. Review.
-
Lifestyle Changes and Industrialization in the Development of Allergic Diseases.Curr Allergy Asthma Rep. 2024 Jul;24(7):331-345. doi: 10.1007/s11882-024-01149-7. Epub 2024 Jun 17. Curr Allergy Asthma Rep. 2024. PMID: 38884832 Free PMC article. Review.
-
Airborne indoor allergen serine proteases and their contribution to sensitisation and activation of innate immunity in allergic airway disease.Eur Respir Rev. 2024 Apr 24;33(172):230126. doi: 10.1183/16000617.0126-2023. Print 2024 Apr 30. Eur Respir Rev. 2024. PMID: 38657996 Free PMC article. Review.
References
-
- Skiepko R., Zietkowski Z., Tomasiak-Lozowska M.M., Tomasiak M., Bodzenta-Lukaszyk A. Bronchial hyperresponsiveness and airway inflammation in patients with seasonal allergic rhinitis. J. Investig. Allergol. Clin. Immunol. 2011;21:532–539. - PubMed
-
- Behrendt H., Ring J. Climate change, environment and allergy. Chem. Immunol. Allergy. 2012;96:7–14. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

