LPS-induced expression and release of monocyte tissue factor in patients with haemophilia
- PMID: 32430703
- PMCID: PMC7316670
- DOI: 10.1007/s00277-020-04075-6
LPS-induced expression and release of monocyte tissue factor in patients with haemophilia
Abstract
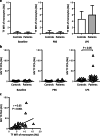
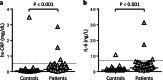
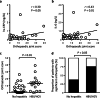
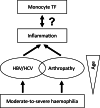
In haemophilia, thrombin generation and fibrin deposition upon vascular injury critically depend on the tissue factor (TF)-driven coagulation pathway. TF expression by monocytes/macrophages and circulating microvesicles contributes to haemostasis, thrombosis and inflammation. Inflammation is a hallmark of blood-induced joint disease. The aim of this study is to correlate TF production by whole-blood monocytes with inflammatory markers and clinical parameters in patients with moderate-to-severe haemophilia A or B (n = 43) in comparison to healthy males (n = 23). Monocyte TF antigen and microvesicle-associated TF procoagulant activity (MV TF PCA) were measured immediately after blood draw (baseline) and following incubation of whole blood with buffer or lipopolysaccharide (LPS) using two-colour flow cytometry and chromogenic FXa generation assay, respectively. Patients with HIV or uncontrolled HBV/HCV infections were excluded. TF was hardly detectable and not different in baseline and buffer-treaded samples from both groups. Stimulation with LPS, however, induced monocyte TF production, with increased TF-specific mean fluorescence intensity (P = 0.08) and MV TF PCA (P < 0.05) in patients compared to controls. Patients also had elevated hs-CRP and IL-6 serum levels (P < 0.001), which correlated with LPS-induced TF parameters. Further exploratory analyses revealed that the presence of systemic (low-grade) inflammation and boosted LPS-induced monocyte TF production were mainly restricted to patients with clinically controlled HBV and/or HCV infection (n = 16), who were older and also had a significantly worse orthopaedic joint score than patients with no history of viral hepatitis (P < 0.01). Our study delineates a previously unrecognised link between systemic inflammation and inducible monocyte TF production in patients with haemophilia A or B.
Keywords: Haemophilia; Hepatitis; Microvesicles; Monocytes; Tissue factor.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Upregulation of monocyte tissue factor activity is significantly associated with low-grade chronic inflammation and insulin resistance in patients with metabolic syndrome.Circ J. 2010 Mar;74(3):572-7. doi: 10.1253/circj.cj-09-0835. Epub 2010 Jan 26. Circ J. 2010. PMID: 20103969
-
Monocyte IL-10 produced in response to lipopolysaccharide modulates thrombin generation by inhibiting tissue factor expression and release of active tissue factor-bound microparticles.Thromb Haemost. 2007 Apr;97(4):598-607. Thromb Haemost. 2007. PMID: 17393023
-
Importance of C-reactive protein in regulating monocyte tissue factor expression in patients with inflammatory rheumatic diseases.J Rheumatol. 2005 Jul;32(7):1224-31. J Rheumatol. 2005. PMID: 15996056
-
The role of platelets in decrypting monocyte tissue factor.Semin Hematol. 2001 Oct;38(4 Suppl 12):2-5. doi: 10.1016/s0037-1963(01)90139-8. Semin Hematol. 2001. PMID: 11735102 Review.
-
Monocyte Tissue Factor Expression: Lipopolysaccharide Induction and Roles in Pathological Activation of Coagulation.Thromb Haemost. 2023 Nov;123(11):1017-1033. doi: 10.1055/a-2091-7006. Epub 2023 May 11. Thromb Haemost. 2023. PMID: 37168007 Free PMC article. Review.
Cited by
-
Cardiovascular Risk in Philadelphia-Negative Myeloproliferative Neoplasms: Mechanisms and Implications-A Narrative Review.Curr Issues Mol Biol. 2024 Aug 2;46(8):8407-8423. doi: 10.3390/cimb46080496. Curr Issues Mol Biol. 2024. PMID: 39194713 Free PMC article. Review.
-
Reduced monocyte proportions and responsiveness in convalescent COVID-19 patients.Front Immunol. 2024 Jan 4;14:1329026. doi: 10.3389/fimmu.2023.1329026. eCollection 2023. Front Immunol. 2024. PMID: 38250080 Free PMC article.
-
Bacitracin and Rutin Regulate Tissue Factor Production in Inflammatory Monocytes and Acute Myeloid Leukemia Blasts.Cancers (Basel). 2021 Aug 4;13(16):3941. doi: 10.3390/cancers13163941. Cancers (Basel). 2021. PMID: 34439096 Free PMC article.
-
Liver cirrhosis and complications from the perspective of dysbiosis.Front Med (Lausanne). 2024 Jan 16;10:1320015. doi: 10.3389/fmed.2023.1320015. eCollection 2023. Front Med (Lausanne). 2024. PMID: 38293307 Free PMC article. Review.
References
-
- Oldenburg J. Optimal treatment strategies for hemophilia: achievements and limitations of current prophylactic regimens. Blood. 2015;125(13):2038–2044. - PubMed
-
- Manco-Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, Ingram JD, Manco-Johnson ML, Funk S, Jacobson L, Valentino LA, Hoots WK, Buchanan GR, DiMichele D, Recht M, Brown D, Leissinger C, Bleak S, Cohen A, Mathew P, Matsunaga A, Medeiros D, Nugent D, Thomas GA, Thompson AA, McRedmond K, Soucie JM, Austin H, Evatt BL. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544. - PubMed
-
- Ahnstrom J, Berntorp E, Lindvall K, Bjorkman S. A 6-year follow-up of dosing, coagulation factor levels and bleedings in relation to joint status in the prophylactic treatment of haemophilia. Haemophilia. 2004;10(6):689–697. - PubMed
-
- Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol. 2018;38(4):709–725. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

