Evaluation of Second-line Anti-VEGF after First-line Anti-EGFR Based Therapy in RAS Wild-Type Metastatic Colorectal Cancer: The Multicenter "SLAVE" Study
- PMID: 32429380
- PMCID: PMC7281759
- DOI: 10.3390/cancers12051259
Evaluation of Second-line Anti-VEGF after First-line Anti-EGFR Based Therapy in RAS Wild-Type Metastatic Colorectal Cancer: The Multicenter "SLAVE" Study
Abstract
: Background: The optimal anti-angiogenic strategy as second-line treatment in RAS wild-type metastatic colorectal cancer (mCRC) treated with anti-EGFR (Epidermal Growth Factor Receptor) based first-line treatment is still debated.
Methods: This multicenter, real-world, retrospective study is aimed at evaluating the effectiveness of second-line Bevacizumab- and Aflibercept-based treatments after an anti-EGFR based first-line regimen. Clinical outcomes measured were: objective response rate (ORR), progression free survival (PFS), overall survival (OS) and adverse events (AEs) profiles.
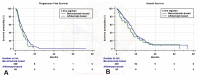
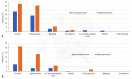
Results: From February 2011 to October 2019, 277 consecutive mCRC patients received Bevacizumab-based (228, 82.3%) or Aflibercept-based (49, 17.7%) regimen. No significant difference was found regarding ORR. The median follow-up was 27.7 months (95%CI: 24.7-34.4). Aflibercept-treated group had a significantly shorter PFS compared to Bevacizumab-treated group (5.6 vs. 7.1 months, respectively) (HR = 1.34 (95%CI: 0.95-1.89); p = 0.0932). The median OS of the Bevacizumab-treated group and Aflibercept-treated group was 16.2 (95%CI: 15.3-18.1) and 12.7 (95%CI: 8.8-17.5) months, respectively (HR= 1.31 (95%CI: 0.89-1.93) p = 0.16). After adjusting for the key covariates (age, gender, performance status, number of metastatic sites and primary tumor side) Bevacizumab-based regimens revealed to be significantly related with a prolonged PFS (HR = 1.44 (95%CI: 1.02-2.03); p = 0.0399) compared to Aflibercept-based regimens, but not with a prolonged OS (HR = 1.47 (95%CI: 0.99-2.17); p = 0.0503). The incidence of G3/G4 VEGF inhibitors class-specific AEs was 7.5% and 26.5% in the Bevacizumab-treated group and the Aflibercept-treated group, respectively (p = 0.0001).
Conclusion: Our analysis seems to reveal that Bevacizumab-based regimens have a slightly better PFS and class-specific AEs profile compared to Aflibercept-based regimen as second-line treatment of RAS wild-type mCRC patients previously treated with anti-EGFR based treatments. These results have to be taken with caution and no conclusive considerations are allowed.
Keywords: Aflibercept; Bevacizumab; Cetuximab; Panitumumab; RAS wild-type mCRC; anti-angiogenics; second-line treatment.
Conflict of interest statement
Alessio Cortellini received grants as a speaker by MSD and Astra-Zeneca, grant consultancies by BMS, Roche, Novartis and Astellas. Dr. Daniele Rossini received personal fees from Takeda. Dr. Ingrid Garajova received grants as a speaker by Amgen and Takeda. The other authors declare no conflict of interest.
Figures
Similar articles
-
Post-Induction Management in Patients With Left-Sided RAS and BRAF Wild-Type Metastatic Colorectal Cancer Treated With First-Line Anti-EGFR-Based Doublet Regimens: A Multicentre Study.Front Oncol. 2021 Oct 27;11:712053. doi: 10.3389/fonc.2021.712053. eCollection 2021. Front Oncol. 2021. PMID: 34778029 Free PMC article.
-
Efficacy of bevacizumab versus epidermal growth factor receptor inhibitors for wild-type RAS metastatic colorectal cancer: a meta-analysis.Onco Targets Ther. 2018 Jul 24;11:4271-4281. doi: 10.2147/OTT.S168695. eCollection 2018. Onco Targets Ther. 2018. PMID: 30100734 Free PMC article.
-
Withholding the Introduction of Anti-Epidermal Growth Factor Receptor: Impact on Outcomes in RAS Wild-Type Metastatic Colorectal Tumors: A Multicenter AGEO Study (the WAIT or ACT Study).Oncologist. 2020 Feb;25(2):e266-e275. doi: 10.1634/theoncologist.2019-0328. Epub 2019 Oct 2. Oncologist. 2020. PMID: 32043796 Free PMC article.
-
Monoclonal antibodies in the treatment of metastatic colorectal cancer: a review.Clin Ther. 2010 Mar;32(3):437-53. doi: 10.1016/j.clinthera.2010.03.012. Clin Ther. 2010. PMID: 20399983 Review.
-
First-line anti-EGFR monoclonal antibodies in panRAS wild-type metastatic colorectal cancer: A systematic review and meta-analysis.Crit Rev Oncol Hematol. 2015 Oct;96(1):156-66. doi: 10.1016/j.critrevonc.2015.05.016. Epub 2015 Jun 5. Crit Rev Oncol Hematol. 2015. PMID: 26088456 Review.
Cited by
-
Real-World Evaluation of Quality of Life, Effectiveness, and Safety of Aflibercept Plus FOLFIRI in Patients with Metastatic Colorectal Cancer: The Prospective QoLiTrap Study.Cancers (Basel). 2022 Jul 20;14(14):3522. doi: 10.3390/cancers14143522. Cancers (Basel). 2022. PMID: 35884583 Free PMC article.
-
Post-Induction Management in Patients With Left-Sided RAS and BRAF Wild-Type Metastatic Colorectal Cancer Treated With First-Line Anti-EGFR-Based Doublet Regimens: A Multicentre Study.Front Oncol. 2021 Oct 27;11:712053. doi: 10.3389/fonc.2021.712053. eCollection 2021. Front Oncol. 2021. PMID: 34778029 Free PMC article.
-
Multimodality Treatment in Metastatic Gastric Cancer: From Past to Next Future.Cancers (Basel). 2020 Sep 11;12(9):2598. doi: 10.3390/cancers12092598. Cancers (Basel). 2020. PMID: 32932914 Free PMC article. Review.
-
Concurrent RAS and RAS/BRAF V600E Variants in Colorectal Cancer: More Frequent Than Expected? A Case Report.Front Oncol. 2022 Apr 7;12:863639. doi: 10.3389/fonc.2022.863639. eCollection 2022. Front Oncol. 2022. PMID: 35463316 Free PMC article.
-
Foxq1 promotes metastasis of nasopharyngeal carcinoma by inducing vasculogenic mimicry via the EGFR signaling pathway.Cell Death Dis. 2021 Apr 19;12(5):411. doi: 10.1038/s41419-021-03674-z. Cell Death Dis. 2021. PMID: 33875643 Free PMC article.
References
-
- Cortellini A., Cannita K., Parisi A., Baldi P.L., Venditti O., D’Orazio C., Dal Mas A., Calvisi G., Giordano A.V., Vicentini V., et al. Weekly alternate intensive regimen FIrB/FOx in metastatic colorectal cancer patients: An update from clinical practice. Onco Targets Ther. 2019;12:2159–2170. doi: 10.2147/OTT.S194745. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous