Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial
- PMID: 32423584
- PMCID: PMC7190303
- DOI: 10.1016/S0140-6736(20)31022-9
Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial
Erratum in
-
Department of Error.Lancet. 2020 May 30;395(10238):1694. doi: 10.1016/S0140-6736(20)31204-6. Lancet. 2020. PMID: 32473675 Free PMC article. No abstract available.
Abstract
Background: No specific antiviral drug has been proven effective for treatment of patients with severe coronavirus disease 2019 (COVID-19). Remdesivir (GS-5734), a nucleoside analogue prodrug, has inhibitory effects on pathogenic animal and human coronaviruses, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in vitro, and inhibits Middle East respiratory syndrome coronavirus, SARS-CoV-1, and SARS-CoV-2 replication in animal models.
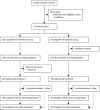
Methods: We did a randomised, double-blind, placebo-controlled, multicentre trial at ten hospitals in Hubei, China. Eligible patients were adults (aged ≥18 years) admitted to hospital with laboratory-confirmed SARS-CoV-2 infection, with an interval from symptom onset to enrolment of 12 days or less, oxygen saturation of 94% or less on room air or a ratio of arterial oxygen partial pressure to fractional inspired oxygen of 300 mm Hg or less, and radiologically confirmed pneumonia. Patients were randomly assigned in a 2:1 ratio to intravenous remdesivir (200 mg on day 1 followed by 100 mg on days 2-10 in single daily infusions) or the same volume of placebo infusions for 10 days. Patients were permitted concomitant use of lopinavir-ritonavir, interferons, and corticosteroids. The primary endpoint was time to clinical improvement up to day 28, defined as the time (in days) from randomisation to the point of a decline of two levels on a six-point ordinal scale of clinical status (from 1=discharged to 6=death) or discharged alive from hospital, whichever came first. Primary analysis was done in the intention-to-treat (ITT) population and safety analysis was done in all patients who started their assigned treatment. This trial is registered with ClinicalTrials.gov, NCT04257656.
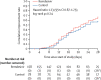
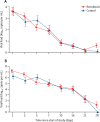
Findings: Between Feb 6, 2020, and March 12, 2020, 237 patients were enrolled and randomly assigned to a treatment group (158 to remdesivir and 79 to placebo); one patient in the placebo group who withdrew after randomisation was not included in the ITT population. Remdesivir use was not associated with a difference in time to clinical improvement (hazard ratio 1·23 [95% CI 0·87-1·75]). Although not statistically significant, patients receiving remdesivir had a numerically faster time to clinical improvement than those receiving placebo among patients with symptom duration of 10 days or less (hazard ratio 1·52 [0·95-2·43]). Adverse events were reported in 102 (66%) of 155 remdesivir recipients versus 50 (64%) of 78 placebo recipients. Remdesivir was stopped early because of adverse events in 18 (12%) patients versus four (5%) patients who stopped placebo early.
Interpretation: In this study of adult patients admitted to hospital for severe COVID-19, remdesivir was not associated with statistically significant clinical benefits. However, the numerical reduction in time to clinical improvement in those treated earlier requires confirmation in larger studies.
Funding: Chinese Academy of Medical Sciences Emergency Project of COVID-19, National Key Research and Development Program of China, the Beijing Science and Technology Project.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Remdesivir for COVID-19: challenges of underpowered studies.Lancet. 2020 May 16;395(10236):1525-1527. doi: 10.1016/S0140-6736(20)31023-0. Epub 2020 Apr 29. Lancet. 2020. PMID: 32423580 Free PMC article. No abstract available.
-
COVID-19 Therapeutics: Making Sense of It All.AACN Adv Crit Care. 2020 Sep 15;31(3):239-249. doi: 10.4037/aacnacc2020792. AACN Adv Crit Care. 2020. PMID: 32668460 No abstract available.
-
Remdesivir and COVID-19: What are the implications for Africa?S Afr Med J. 2020 May 7;110(6):12942. S Afr Med J. 2020. PMID: 32880546 No abstract available.
-
Remdesivir and COVID-19.Lancet. 2020 Oct 3;396(10256):952. doi: 10.1016/S0140-6736(20)32021-3. Lancet. 2020. PMID: 33010831 Free PMC article. No abstract available.
-
Remdesivir and COVID-19.Lancet. 2020 Oct 3;396(10256):953. doi: 10.1016/S0140-6736(20)32020-1. Lancet. 2020. PMID: 33010832 Free PMC article. No abstract available.
-
Remdesivir and COVID-19.Lancet. 2020 Oct 3;396(10256):953-954. doi: 10.1016/S0140-6736(20)32019-5. Lancet. 2020. PMID: 33010833 Free PMC article. No abstract available.
-
The place for remdesivir in COVID-19 treatment.Lancet Infect Dis. 2021 Jan;21(1):20-21. doi: 10.1016/S1473-3099(20)30911-7. Epub 2020 Nov 26. Lancet Infect Dis. 2021. PMID: 33248473 Free PMC article. No abstract available.
Similar articles
-
Evaluation of the efficacy and safety of intravenous remdesivir in adult patients with severe COVID-19: study protocol for a phase 3 randomized, double-blind, placebo-controlled, multicentre trial.Trials. 2020 May 24;21(1):422. doi: 10.1186/s13063-020-04352-9. Trials. 2020. PMID: 32448345 Free PMC article.
-
Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial.JAMA. 2020 Sep 15;324(11):1048-1057. doi: 10.1001/jama.2020.16349. JAMA. 2020. PMID: 32821939 Free PMC article. Clinical Trial.
-
Remdesivir for the Treatment of Covid-19 - Final Report.N Engl J Med. 2020 Nov 5;383(19):1813-1826. doi: 10.1056/NEJMoa2007764. Epub 2020 Oct 8. N Engl J Med. 2020. PMID: 32445440 Free PMC article. Clinical Trial.
-
Compounds with Therapeutic Potential against Novel Respiratory 2019 Coronavirus.Antimicrob Agents Chemother. 2020 Apr 21;64(5):e00399-20. doi: 10.1128/AAC.00399-20. Print 2020 Apr 21. Antimicrob Agents Chemother. 2020. PMID: 32152082 Free PMC article. Review.
-
Remdesivir in COVID-19: A critical review of pharmacology, pre-clinical and clinical studies.Diabetes Metab Syndr. 2020 Jul-Aug;14(4):641-648. doi: 10.1016/j.dsx.2020.05.018. Epub 2020 May 12. Diabetes Metab Syndr. 2020. PMID: 32428865 Free PMC article. Review.
Cited by
-
GPCR Inhibitors Have Antiviral Properties against JC Polyomavirus Infection.Viruses. 2024 Sep 30;16(10):1559. doi: 10.3390/v16101559. Viruses. 2024. PMID: 39459893 Free PMC article.
-
Limited Short-Term Evolution of SARS-CoV-2 RNA-Dependent RNA Polymerase under Remdesivir Exposure in Upper Respiratory Compartments.Viruses. 2024 Sep 24;16(10):1511. doi: 10.3390/v16101511. Viruses. 2024. PMID: 39459846 Free PMC article.
-
The Effectiveness and Safety of Remdesivir Use in COVID-19 Patients with Neutropenia: A Retrospective Cohort Study.Life (Basel). 2024 Oct 1;14(10):1252. doi: 10.3390/life14101252. Life (Basel). 2024. PMID: 39459552 Free PMC article.
-
Multidrug Combinations against SARS-CoV-2 Using GS-441524 or Ivermectin with Molnupiravir and/or Nirmatrelvir in Reconstituted Human Nasal Airway Epithelia.Pharmaceutics. 2024 Sep 27;16(10):1262. doi: 10.3390/pharmaceutics16101262. Pharmaceutics. 2024. PMID: 39458594 Free PMC article.
-
Comparative clinical trial of Langenlianqiao oral liquid and Lianhuaqingwen capsule in the treatment of mild cases of coronavirus disease 2019 (COVID-19).J Health Popul Nutr. 2024 Oct 12;43(1):158. doi: 10.1186/s41043-024-00649-6. J Health Popul Nutr. 2024. PMID: 39396047 Free PMC article. Clinical Trial.
References
-
- Johns Hopkins University and Medicine COVID-19 map. Johns Hopkins Coronavirus Resource Centre. https://coronavirus.jhu.edu/map.html
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

