Risks and Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin-Receptor Blockers on SARS-CoV-2 Infection in Adults: A Living Systematic Review
- PMID: 32422062
- PMCID: PMC7249560
- DOI: 10.7326/M20-1515
Risks and Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin-Receptor Blockers on SARS-CoV-2 Infection in Adults: A Living Systematic Review
Update in
-
Update Alert 7: Risks and Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin-Receptor Blockers on SARS-CoV-2 Infection in Adults.Ann Intern Med. 2021 Feb;174(2):W25-W29. doi: 10.7326/L20-1446. Epub 2021 Jan 5. Ann Intern Med. 2021. PMID: 33395346 Free PMC article. No abstract available.
-
Update Alert 8: Risks and Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin-Receptor Blockers on SARS-CoV-2 Infection in Adults.Ann Intern Med. 2021 Jun;174(6):W54-W55. doi: 10.7326/L21-0223. Epub 2021 Apr 27. Ann Intern Med. 2021. PMID: 33900794 Free PMC article. No abstract available.
Abstract
Background: The role of angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin-receptor blockers (ARBs) in coronavirus disease 2019 (COVID-19) susceptibility, severity, and treatment is unclear.
Purpose: To evaluate, on an ongoing basis, whether use of ACEIs or ARBs either increases risk for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or is associated with worse COVID-19 disease outcomes, and to assess the efficacy of these medications for COVID-19 treatment.
Data sources: MEDLINE (Ovid) and Cochrane Database of Systematic Reviews from 2003 to 4 May 2020, with planned ongoing surveillance for 1 year; the World Health Organization database of COVID-19 publications and medRxiv.org through 17 April 2020; and ClinicalTrials.gov to 24 April 2020, with planned ongoing surveillance.
Study selection: Observational studies and trials in adults that examined associations and effects of ACEIs or ARBs on risk for SARS-CoV-2 infection and COVID-19 disease severity and mortality.
Data extraction: Single-reviewer abstraction confirmed by another reviewer, independent evaluation by 2 reviewers of study quality, and collective assessment of certainty of evidence.
Data synthesis: Two retrospective cohort studies found that ACEI and ARB use was not associated with a higher likelihood of receiving a positive SARS-CoV-2 test result, and 1 case-control study found no association with COVID-19 illness in a large community (moderate-certainty evidence). Fourteen observational studies, involving a total of 23 565 adults with COVID-19, showed consistent evidence that neither medication was associated with more severe COVID-19 illness (high-certainty evidence). Four registered randomized trials plan to evaluate ACEIs and ARBs for treatment of COVID-19.
Limitation: Half the studies were small and did not adjust for important confounding variables.
Conclusion: High-certainty evidence suggests that ACEI or ARB use is not associated with more severe COVID-19 disease, and moderate-certainty evidence suggests no association between use of these medications and positive SARS-CoV-2 test results among symptomatic patients. Whether these medications increase the risk for mild or asymptomatic disease or are beneficial in COVID-19 treatment remains uncertain.
Primary funding source: None. (PROSPERO: registration number pending).
Figures
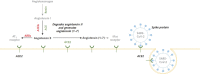
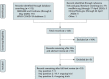
Comment in
-
COVID-19 and Angiotensin-Converting Enzyme Inhibitor/Angiotensin-Receptor Blocker Therapy.Ann Intern Med. 2020 Aug 4;173(3):237-238. doi: 10.7326/M20-3047. Epub 2020 May 15. Ann Intern Med. 2020. PMID: 32422077 Free PMC article.
-
Update Alert: Risks and Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin-Receptor Blockers on SARS-CoV-2 Infection in Adults.Ann Intern Med. 2020 Aug 4;173(3):W66. doi: 10.7326/L20-0887. Epub 2020 Jun 25. Ann Intern Med. 2020. PMID: 32584593 Free PMC article. No abstract available.
-
Update Alert 2: Risks and Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin-Receptor Blockers on SARS-CoV-2 Infection in Adults.Ann Intern Med. 2020 Sep 1;173(5):W87. doi: 10.7326/L20-0969. Epub 2020 Jul 23. Ann Intern Med. 2020. PMID: 32701362 Free PMC article. No abstract available.
-
Update Alert 3: Risks and Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin-Receptor Blockers on SARS-CoV-2 Infection in Adults.Ann Intern Med. 2020 Oct 6;173(7):130-131. doi: 10.7326/L20-1068. Epub 2020 Aug 26. Ann Intern Med. 2020. PMID: 32845705 Free PMC article. No abstract available.
-
Update Alert 4: Risks and Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin-Receptor Blockers on SARS-CoV-2 Infection in Adults.Ann Intern Med. 2020 Nov 3;173(9):W147-W148. doi: 10.7326/L20-1177. Epub 2020 Sep 22. Ann Intern Med. 2020. PMID: 32956599 Free PMC article. No abstract available.
-
Update Alert 5: Risks and Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin-Receptor Blockers on SARS-CoV-2 Infection in Adults.Ann Intern Med. 2020 Dec 15;173(12):167-168. doi: 10.7326/L20-1293. Epub 2020 Oct 27. Ann Intern Med. 2020. PMID: 33105094 Free PMC article. No abstract available.
-
Update Alert 6: Risks and Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin-Receptor Blockers on SARS-CoV-2 Infection in Adults.Ann Intern Med. 2021 Jan;174(1):W17. doi: 10.7326/L20-1355. Epub 2020 Dec 1. Ann Intern Med. 2021. PMID: 33253036 Free PMC article. No abstract available.
-
Update Alert 9: Risks and Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin-Receptor Blockers on SARS-CoV-2 Infection in Adults.Ann Intern Med. 2022 Apr;175(4):W47-W48. doi: 10.7326/L21-0791. Epub 2022 Feb 8. Ann Intern Med. 2022. PMID: 35130048 Free PMC article. No abstract available.
-
Update Alert 10: Risks and Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin-Receptor Blockers on SARS-CoV-2 Infection in Adults.Ann Intern Med. 2023 May;176(5):eL230049. doi: 10.7326/L23-0049. Epub 2023 Mar 21. Ann Intern Med. 2023. PMID: 36940439 Free PMC article. No abstract available.
Similar articles
-
Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors-lessons from available evidence and insights into COVID-19.Hypertens Res. 2020 Jul;43(7):648-654. doi: 10.1038/s41440-020-0455-8. Epub 2020 Apr 27. Hypertens Res. 2020. PMID: 32341442 Free PMC article. Review.
-
A systematic review and meta-analysis of the use of renin-angiotensin system drugs and COVID-19 clinical outcomes: What is the evidence so far?Pharmacol Res Perspect. 2020 Dec;8(6):e00666. doi: 10.1002/prp2.666. Pharmacol Res Perspect. 2020. PMID: 33084232 Free PMC article.
-
Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers and the Risk of SARS-CoV-2 Infection or Hospitalization With COVID-19 Disease: A Systematic Review and Meta-Analysis.Am J Ther. 2020 Dec 28;29(1):e74-e84. doi: 10.1097/MJT.0000000000001319. Am J Ther. 2020. PMID: 33395057
-
Association of Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers With Testing Positive for Coronavirus Disease 2019 (COVID-19).JAMA Cardiol. 2020 Sep 1;5(9):1020-1026. doi: 10.1001/jamacardio.2020.1855. JAMA Cardiol. 2020. PMID: 32936273 Free PMC article.
-
Risks of ACE Inhibitor and ARB Usage in COVID-19: Evaluating the Evidence.Clin Pharmacol Ther. 2020 Aug;108(2):236-241. doi: 10.1002/cpt.1863. Epub 2020 May 10. Clin Pharmacol Ther. 2020. PMID: 32320478 Free PMC article. Review.
Cited by
-
Association of antihypertensive drugs with COVID-19 outcomes: a drug-target Mendelian randomization study.Front Pharmacol. 2023 Dec 5;14:1224737. doi: 10.3389/fphar.2023.1224737. eCollection 2023. Front Pharmacol. 2023. PMID: 38116083 Free PMC article.
-
COVID-19 and cardiovascular disease in patients with chronic kidney disease.Nephrol Dial Transplant. 2024 Jan 31;39(2):177-189. doi: 10.1093/ndt/gfad170. Nephrol Dial Transplant. 2024. PMID: 37771078 Free PMC article. Review.
-
Ramipril for the Treatment of COVID-19: RAMIC, a Randomized, Double-Blind, Placebo-Controlled Clinical Trial.Adv Ther. 2023 Nov;40(11):4805-4816. doi: 10.1007/s12325-023-02618-7. Epub 2023 Aug 24. Adv Ther. 2023. PMID: 37615850 Free PMC article. Clinical Trial.
-
Methodological quality and reporting quality of COVID-19 living systematic review: a cross-sectional study.BMC Med Res Methodol. 2023 Jul 31;23(1):175. doi: 10.1186/s12874-023-01980-y. BMC Med Res Methodol. 2023. PMID: 37525117 Free PMC article.
-
Learning and Investigation of the Role of Angiotensin-Converting Enzyme in Radiotherapy for Nasopharyngeal Carcinoma.Biomedicines. 2023 May 30;11(6):1581. doi: 10.3390/biomedicines11061581. Biomedicines. 2023. PMID: 37371679 Free PMC article.
References
-
- Watkins J. Preventing a covid-19 pandemic [Editorial]. BMJ. 2020;368:m810. [PMID: 32111649] doi:10.1136/bmj.m810. - PubMed
-
- Gauer R, LaRocque J. JNC 8: relaxing the standards [Editorial]. Am Fam Physician. 2014;90:449-52. [PMID: 25369620] - PubMed
-
- American Diabetes Association. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S111-S134. [PMID: 31862753] doi:10.2337/dc20-S010. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
