Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19)
- PMID: 32418199
- PMCID: PMC7276767
- DOI: 10.1002/path.5471
Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19)
Abstract
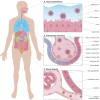
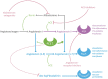
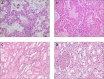
Angiotensin-converting enzyme 2 (ACE2) has been established as the functional host receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for the current devastating worldwide pandemic of coronavirus disease 2019 (COVID-19). ACE2 is abundantly expressed in a variety of cells residing in many different human organs. In human physiology, ACE2 is a pivotal counter-regulatory enzyme to ACE by the breakdown of angiotensin II, the central player in the renin-angiotensin-aldosterone system (RAAS) and the main substrate of ACE2. Many factors have been associated with both altered ACE2 expression and COVID-19 severity and progression, including age, sex, ethnicity, medication, and several co-morbidities, such as cardiovascular disease and metabolic syndrome. Although ACE2 is widely distributed in various human tissues and many of its determinants have been well recognised, ACE2-expressing organs do not equally participate in COVID-19 pathophysiology, implying that other mechanisms are involved in orchestrating cellular infection resulting in tissue damage. Reports of pathologic findings in tissue specimens of COVID-19 patients are rapidly emerging and confirm the established role of ACE2 expression and activity in disease pathogenesis. Identifying pathologic changes caused by SARS-CoV-2 infection is crucially important as it has major implications for understanding COVID-19 pathophysiology and the development of evidence-based treatment strategies. Currently, many interventional strategies are being explored in ongoing clinical trials, encompassing many drug classes and strategies, including antiviral drugs, biological response modifiers, and RAAS inhibitors. Ultimately, prevention is key to combat COVID-19 and appropriate measures are being taken accordingly, including development of effective vaccines. In this review, we describe the role of ACE2 in COVID-19 pathophysiology, including factors influencing ACE2 expression and activity in relation to COVID-19 severity. In addition, we discuss the relevant pathological changes resulting from SARS-CoV-2 infection. Finally, we highlight a selection of potential treatment modalities for COVID-19. © 2020 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Keywords: angiotensin-converting enzyme 2 (ACE2); coronavirus disease 2019 (COVID-19); organ involvement; pathology; pathophysiology; renin-angiotensin-aldosterone system (RAAS); risk factors; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); treatment.
© 2020 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Figures

Similar articles
-
SARS-CoV-2 pandemic and research gaps: Understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy.Theranostics. 2020 Jun 12;10(16):7448-7464. doi: 10.7150/thno.48076. eCollection 2020. Theranostics. 2020. PMID: 32642005 Free PMC article. Review.
-
Endocrine aspects of ACE2 regulation: RAAS, steroid hormones and SARS-CoV-2.J Endocrinol. 2020 Nov;247(2):R45-R62. doi: 10.1530/JOE-20-0260. J Endocrinol. 2020. PMID: 32966970 Review.
-
Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2.Circ Res. 2020 May 8;126(10):1456-1474. doi: 10.1161/CIRCRESAHA.120.317015. Epub 2020 Apr 8. Circ Res. 2020. PMID: 32264791 Free PMC article. Review.
-
SARS-CoV-2 receptor ACE2-dependent implications on the cardiovascular system: From basic science to clinical implications.J Mol Cell Cardiol. 2020 Jul;144:47-53. doi: 10.1016/j.yjmcc.2020.04.031. Epub 2020 Apr 30. J Mol Cell Cardiol. 2020. PMID: 32360703 Free PMC article. Review.
-
Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19.J Med Virol. 2020 Jul;92(7):726-730. doi: 10.1002/jmv.25785. Epub 2020 Apr 5. J Med Virol. 2020. PMID: 32221983 Free PMC article. Review.
Cited by
-
Increased circulating microparticles contribute to severe infection and adverse outcomes of COVID-19 in patients with diabetes.Am J Physiol Heart Circ Physiol. 2022 Dec 1;323(6):H1176-H1193. doi: 10.1152/ajpheart.00409.2022. Epub 2022 Oct 21. Am J Physiol Heart Circ Physiol. 2022. PMID: 36269646 Free PMC article.
-
Corticosteroids for COVID-19 Therapy: Potential Implications on Tuberculosis.Int J Mol Sci. 2021 Apr 6;22(7):3773. doi: 10.3390/ijms22073773. Int J Mol Sci. 2021. PMID: 33917321 Free PMC article. Review.
-
Level up for culture models - How 3D cell culture models benefit SARS-CoV-2 research.Biomed J. 2021 Mar;44(1):1-6. doi: 10.1016/j.bj.2021.02.001. Epub 2021 Feb 9. Biomed J. 2021. PMID: 33741318 Free PMC article.
-
A comprehensive review on disposition kinetics and dosage of oral administration of Andrographis paniculata, an alternative herbal medicine, in co-treatment of coronavirus disease.Front Pharmacol. 2022 Aug 19;13:952660. doi: 10.3389/fphar.2022.952660. eCollection 2022. Front Pharmacol. 2022. PMID: 36059950 Free PMC article. Review.
-
Nutritional status of patients with COVID-19.Int J Infect Dis. 2020 Nov;100:390-393. doi: 10.1016/j.ijid.2020.08.018. Epub 2020 Aug 11. Int J Infect Dis. 2020. PMID: 32795605 Free PMC article.
References
-
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323 : 1239–1242. - PubMed
-
- Report of the WHO–China Joint Mission on Coronavirus Disease 2019 (COVID‐19), 16–24 February 2020 . [Accessed 7 April 2020]. Available from: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mis...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

