Progranulin/EphA2 axis: A novel oncogenic mechanism in bladder cancer
- PMID: 32417448
- PMCID: PMC8162889
- DOI: 10.1016/j.matbio.2020.03.009
Progranulin/EphA2 axis: A novel oncogenic mechanism in bladder cancer
Abstract
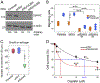
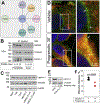
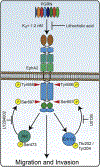
The growth factor progranulin plays a critical role in bladder cancer by modulating tumor cell motility and invasion. Progranulin regulates remodeling of the actin cytoskeleton by interacting with drebrin, an actin binding protein that regulates tumor growth. We previously discovered that progranulin depletion inhibits epithelial-to-mesenchymal transition and markedly reduces in vivo tumor growth. Moreover, progranulin depletion sensitizes urothelial cancer cells to cisplatin treatment, further substantiating a pro-survival function of progranulin. Until recently, the progranulin signaling receptor remained unidentified, precluding a full understanding of progranulin action in tumor cell biology. We recently identified EphA2, a member of a large family of receptor tyrosine-kinases, as the functional receptor for progranulin. However, it is not established whether EphA2 plays an oncogenic role in bladder cancer. Here we demonstrate that progranulin, and not ephrin-A1, the canonical ligand for EphA2, is the predominant EphA2 ligand in bladder cancer. Progranulin evoked Akt- and Erk1/2-mediated EphA2 phosphorylation at Ser897, which could drive bladder tumorigenesis. We discovered that EphA2 depletion severely blunted progranulin-dependent motility and anchorage-independent growth, and sensitized bladder cancer cells to cisplatin treatment. We further defined the mechanisms of progranulin/EphA2-dependent motility by identifying liprin-α1 as a novel progranulin-dependent EphA2 interacting protein and establishing its critical role in cell motility. The discovery of EphA2 as the functional signaling receptor for progranulin and the identification of novel downstream effectors offer a new avenue for understanding the underlying mechanism of progranulin action and may constitute novel clinical and therapeutic targets in bladder cancer.
Keywords: Bladder cancer; EphA2; Liprinα-1; Motility; Progranulin.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Disclosure
The authors declare no competing financial interests.
Figures


Similar articles
-
Suppression of progranulin expression inhibits bladder cancer growth and sensitizes cancer cells to cisplatin.Oncotarget. 2016 Jun 28;7(26):39980-39995. doi: 10.18632/oncotarget.9556. Oncotarget. 2016. PMID: 27220888 Free PMC article.
-
A novel role for drebrin in regulating progranulin bioactivity in bladder cancer.Oncotarget. 2015 May 10;6(13):10825-39. doi: 10.18632/oncotarget.3424. Oncotarget. 2015. PMID: 25839164 Free PMC article.
-
Complexity of progranulin mechanisms of action in mesothelioma.J Exp Clin Cancer Res. 2022 Dec 5;41(1):333. doi: 10.1186/s13046-022-02546-4. J Exp Clin Cancer Res. 2022. PMID: 36471440 Free PMC article.
-
Emerging and Diverse Functions of the EphA2 Noncanonical Pathway in Cancer Progression.Biol Pharm Bull. 2017;40(10):1616-1624. doi: 10.1248/bpb.b17-00446. Biol Pharm Bull. 2017. PMID: 28966234 Review.
-
Progranulin Oncogenic Network in Solid Tumors.Cancers (Basel). 2023 Mar 10;15(6):1706. doi: 10.3390/cancers15061706. Cancers (Basel). 2023. PMID: 36980592 Free PMC article. Review.
Cited by
-
Development and validation of prognostic index based on purine metabolism genes in patients with bladder cancer.Front Med (Lausanne). 2023 Sep 14;10:1193133. doi: 10.3389/fmed.2023.1193133. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37780567 Free PMC article.
-
IRE1α protects against osteoarthritis by regulating progranulin-dependent XBP1 splicing and collagen homeostasis.Exp Mol Med. 2023 Nov;55(11):2376-2389. doi: 10.1038/s12276-023-01106-w. Epub 2023 Nov 1. Exp Mol Med. 2023. PMID: 37907740 Free PMC article.
-
Homocysteine Impairs Endothelial Cell Barrier Function and Angiogenic Potential via the Progranulin/EphA2 Pathway.Front Pharmacol. 2021 Jan 8;11:614760. doi: 10.3389/fphar.2020.614760. eCollection 2020. Front Pharmacol. 2021. PMID: 33510642 Free PMC article.
-
Eph receptors and ephrins in cancer progression.Nat Rev Cancer. 2024 Jan;24(1):5-27. doi: 10.1038/s41568-023-00634-x. Epub 2023 Nov 23. Nat Rev Cancer. 2024. PMID: 37996538 Free PMC article. Review.
-
Comprehensive analysis of Epha10 as a predictor of clinical prognosis and immune checkpoint therapy efficacy in non-small cell lung cancer.Sci Rep. 2024 Aug 23;14(1):19623. doi: 10.1038/s41598-024-70466-8. Sci Rep. 2024. PMID: 39179608 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A, Cancer statistics, 2019, 69(1) (2019) 7–34. - PubMed
-
- Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R, The health economics of bladder cancer: a comprehensive review of the published literature, Pharmacoeconomics 21 (18) (2003) 1315–1330. - PubMed
-
- Lee SH, Hu W, Matulay JT, Silva MV, Owczarek TB, Kim K, Chua CW, Barlow LJ, Kandoth C, Williams AB, Bergren SK, Pietzak EJ, Anderson CB, Benson MC, Coleman JA, Taylor BS, Abate-Shen C, McKiernan JM, Al-Ahmadie H, Solit DB, Shen MM, Tumor evolution and drug response in patient-derived organoid models of bladder cancer, Cell 173 (2) (2018) 515–528. - PMC - PubMed
-
- Bladder cancer: diagnosis and management of bladder cancer: (c) NICE (2015) Bladder cancer: diagnosis and management of bladder cancer, BJU Int. 120 (6) (2017) 755–765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

