Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis
- PMID: 32409522
- PMCID: PMC7828900
- DOI: 10.1503/cmaj.200645
Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis
Abstract
Background: Very little direct evidence exists on use of corticosteroids in patients with coronavirus disease 2019 (COVID-19). Indirect evidence from related conditions must therefore inform inferences regarding benefits and harms. To support a guideline for managing COVID-19, we conducted systematic reviews examining the impact of corticosteroids in COVID-19 and related severe acute respiratory illnesses.
Methods: We searched standard international and Chinese biomedical literature databases and prepublication sources for randomized controlled trials (RCTs) and observational studies comparing corticosteroids versus no corticosteroids in patients with COVID-19, severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS). For acute respiratory distress syndrome (ARDS), influenza and community-acquired pneumonia (CAP), we updated the most recent rigorous systematic review. We conducted random-effects meta-analyses to pool relative risks and then used baseline risk in patients with COVID-19 to generate absolute effects.
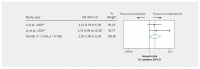
Results: In ARDS, according to 1 small cohort study in patients with COVID-19 and 7 RCTs in non-COVID-19 populations (risk ratio [RR] 0.72, 95% confidence interval [CI] 0.55 to 0.93, mean difference 17.3% fewer; low-quality evidence), corticosteroids may reduce mortality. In patients with severe COVID-19 but without ARDS, direct evidence from 2 observational studies provided very low-quality evidence of an increase in mortality with corticosteroids (hazard ratio [HR] 2.30, 95% CI 1.00 to 5.29, mean difference 11.9% more), as did observational data from influenza studies. Observational data from SARS and MERS studies provided very low-quality evidence of a small or no reduction in mortality. Randomized controlled trials in CAP suggest that corticosteroids may reduce mortality (RR 0.70, 95% CI 0.50 to 0.98, 3.1% lower; very low-quality evidence), and may increase hyperglycemia.
Interpretation: Corticosteroids may reduce mortality for patients with COVID-19 and ARDS. For patients with severe COVID-19 but without ARDS, evidence regarding benefit from different bodies of evidence is inconsistent and of very low quality.
© 2020 Joule Inc. or its licensors.
Conflict of interest statement
Competing interests: Bram Rochwerg is an investigator in a trial, supported by a Canadian Institute of Health Research grant, evaluating the effect of corticosteroids in COVID-19 patients. No other competing interests were declared.
Figures




Comment in
- CMAJ. 192:E536.
Similar articles
-
Efficacy and safety of convalescent plasma for severe COVID-19 based on evidence in other severe respiratory viral infections: a systematic review and meta-analysis.CMAJ. 2020 Jul 6;192(27):E745-E755. doi: 10.1503/cmaj.200642. Epub 2020 May 22. CMAJ. 2020. PMID: 32444482 Free PMC article.
-
Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis.Leukemia. 2020 Jun;34(6):1503-1511. doi: 10.1038/s41375-020-0848-3. Epub 2020 May 5. Leukemia. 2020. PMID: 32372026 Free PMC article.
-
Efficacy and safety of antiviral treatment for COVID-19 from evidence in studies of SARS-CoV-2 and other acute viral infections: a systematic review and meta-analysis.CMAJ. 2020 Jul 6;192(27):E734-E744. doi: 10.1503/cmaj.200647. Epub 2020 Jun 3. CMAJ. 2020. PMID: 32493740 Free PMC article.
-
Corticosteroid use in viral pneumonia: experience so far and the dexamethasone breakthrough in coronavirus disease-2019.J Comp Eff Res. 2020 Dec;9(18):1247-1254. doi: 10.2217/cer-2020-0146. Epub 2020 Nov 27. J Comp Eff Res. 2020. PMID: 33245242 Free PMC article. Review.
-
Corticosteroid administration for viral pneumonia: COVID-19 and beyond.Clin Microbiol Infect. 2020 Sep;26(9):1171-1177. doi: 10.1016/j.cmi.2020.06.020. Epub 2020 Jun 27. Clin Microbiol Infect. 2020. PMID: 32603802 Free PMC article. Review.
Cited by
-
The relationship between pragmatism, timing, and study size on impact of randomized trials: a qualitative, hypothesis generating study of trials of systemic corticosteroids for COVID-19.J Clin Epidemiol. 2022 Dec;152:116-124. doi: 10.1016/j.jclinepi.2022.09.018. Epub 2022 Oct 6. J Clin Epidemiol. 2022. PMID: 36209914 Free PMC article. Review.
-
SGLT2 Inhibitors in Long COVID Syndrome: Is There a Potential Role?J Cardiovasc Dev Dis. 2023 Nov 29;10(12):478. doi: 10.3390/jcdd10120478. J Cardiovasc Dev Dis. 2023. PMID: 38132646 Free PMC article. Review.
-
Acute Exacerbation of Interstitial Lung Disease as a Sequela of COVID-19 Pneumonia.Am J Med Sci. 2021 Jan;361(1):126-129. doi: 10.1016/j.amjms.2020.08.017. Epub 2020 Aug 11. Am J Med Sci. 2021. PMID: 32912600 Free PMC article. No abstract available.
-
Dexamethasone in the era of COVID-19: friend or foe? An essay on the effects of dexamethasone and the potential risks of its inadvertent use in patients with diabetes.Diabetol Metab Syndr. 2020 Sep 7;12:80. doi: 10.1186/s13098-020-00583-7. eCollection 2020. Diabetol Metab Syndr. 2020. PMID: 32922517 Free PMC article. Review.
-
Corticosteroids: A Controversial Therapy for Coronavirus Disease 2019.J Transl Int Med. 2020 Sep 25;8(3):115-118. doi: 10.2478/jtim-2020-0019. eCollection 2020 Sep. J Transl Int Med. 2020. PMID: 33062586 Free PMC article. No abstract available.
References
-
- WHO Director-General’s opening remarks at the media briefing on COVID-19 — 11 March 2020. Geneva: World Health Organization; 2020. Available: www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at... (accessed 2020 Apr. 23).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
