Advantages of Using Paclitaxel in Combination with Oncolytic Adenovirus Utilizing RNA Destabilization Mechanism
- PMID: 32408515
- PMCID: PMC7281177
- DOI: 10.3390/cancers12051210
Advantages of Using Paclitaxel in Combination with Oncolytic Adenovirus Utilizing RNA Destabilization Mechanism
Abstract
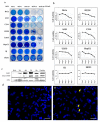
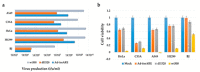
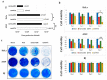
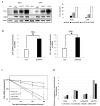
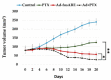
Oncolytic virotherapy is a novel approach to cancer therapy. Ad-fosARE is a conditionally replicative adenovirus engineered by inserting AU-rich elements (ARE) in the 3'-untranslated region of the E1A gene. In this study, we examined the oncolytic activity of Ad-fosARE and used it in a synergistic combination with the chemotherapeutic agent paclitaxel (PTX) for treating cancer cells. The expression of E1A was high in cancer cells due to stabilized E1A-ARE mRNA. As a result, the efficiency of its replication and cytolytic activity in cancer cells was higher than in normal cells. PTX treatment increased the cytoplasmic HuR relocalization in cancer cells, enhanced viral replication through elevated E1A expression, and upregulated CAR (Coxsackie-adenovirus receptor) required for viral uptake. Furthermore, PTX altered the instability of microtubules by acetylation and detyrosination, which is essential for viral internalization and trafficking to the nucleus. These results indicate that PTX can provide multiple advantages to the efficacy of Ad-fosARE both in vitro and in vivo, and provides a basis for designing novel clinical trials. Thus, this virus has a lot of benefits that are not found in other oncolytic viruses. The virus also has the potential for treating PXT-resistant cancers.
Keywords: ARE-mRNA; AU-rich element (ARE), HuR; CAR; E1A; Microtubules; Oncolytic adenovirus; Paclitaxel.
Conflict of interest statement
No conflict of interest.
Figures



Similar articles
-
Enhanced oncolytic activity of E4orf6-deficient adenovirus by facilitating nuclear export of HuR.Biochem Biophys Res Commun. 2020 Aug 20;529(2):494-499. doi: 10.1016/j.bbrc.2020.04.147. Epub 2020 Jul 2. Biochem Biophys Res Commun. 2020. PMID: 32703457
-
Conditionally Replicative Adenovirus Controlled by the Stabilization System of AU-Rich Elements Containing mRNA.Cancers (Basel). 2020 May 11;12(5):1205. doi: 10.3390/cancers12051205. Cancers (Basel). 2020. PMID: 32403262 Free PMC article.
-
Synergistic antitumor effect mediated by a paclitaxel-conjugated polymeric micelle-coated oncolytic adenovirus.Biomaterials. 2017 Nov;145:207-222. doi: 10.1016/j.biomaterials.2017.08.035. Epub 2017 Aug 23. Biomaterials. 2017. PMID: 28869866
-
Etoposide enhances antitumor efficacy of MDR1-driven oncolytic adenovirus through autoupregulation of the MDR1 promoter activity.Oncotarget. 2015 Nov 10;6(35):38308-26. doi: 10.18632/oncotarget.5702. Oncotarget. 2015. PMID: 26515462 Free PMC article.
-
Recombinant adenovirus with enhanced green fluorescent protein.2007 Dec 9 [updated 2008 Jan 2]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2007 Dec 9 [updated 2008 Jan 2]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641460 Free Books & Documents. Review.
Cited by
-
Oncolytic vesicular stomatitis virus alone or in combination with JAK inhibitors is effective against ovarian cancer.Mol Ther Oncol. 2024 Jun 8;32(3):200826. doi: 10.1016/j.omton.2024.200826. eCollection 2024 Sep 19. Mol Ther Oncol. 2024. PMID: 39006945 Free PMC article.
-
Human adenovirus oncolytic properties and the inhibitory role of E4 orf4 and E4 orf6/7 on endogenously activated NF-κB.Biochem Biophys Rep. 2023 Dec 22;37:101616. doi: 10.1016/j.bbrep.2023.101616. eCollection 2024 Mar. Biochem Biophys Rep. 2023. PMID: 38205184 Free PMC article.
-
Oncolytic virotherapy reverses the immunosuppressive tumor microenvironment and its potential in combination with immunotherapy.Cancer Cell Int. 2021 May 13;21(1):262. doi: 10.1186/s12935-021-01972-2. Cancer Cell Int. 2021. PMID: 33985527 Free PMC article. Review.
-
Interferon-expressing oncolytic adenovirus + chemoradiation inhibited pancreatic cancer growth in a hamster model.Cancer Sci. 2023 Sep;114(9):3759-3769. doi: 10.1111/cas.15903. Epub 2023 Jul 13. Cancer Sci. 2023. PMID: 37439437 Free PMC article.
-
Evolving Role of Oncolytic Virotherapy: Challenges and Prospects in Clinical Practice.JCO Precis Oncol. 2021 Feb 24;5:PO.20.00395. doi: 10.1200/PO.20.00395. eCollection 2021. JCO Precis Oncol. 2021. PMID: 34250386 Free PMC article. Review.
References
-
- Abou El Hassan M.A., Van der Meulen-Muileman I., Abbas S., Kruyt F.A. Conditionally replicating adenoviruses kill tumor cells via a basic apoptotic machinery—independent mechanism that resembles necrosis-like programmed cell death. J. Virol. 2004;78:12243–12251. doi: 10.1128/JVI.78.22.12243-12251.2004. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

