Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial
- PMID: 32401715
- PMCID: PMC7211500
- DOI: 10.1016/S0140-6736(20)31042-4
Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial
Abstract
Background: Effective antiviral therapy is important for tackling the coronavirus disease 2019 (COVID-19) pandemic. We assessed the efficacy and safety of combined interferon beta-1b, lopinavir-ritonavir, and ribavirin for treating patients with COVID-19.
Methods: This was a multicentre, prospective, open-label, randomised, phase 2 trial in adults with COVID-19 who were admitted to six hospitals in Hong Kong. Patients were randomly assigned (2:1) to a 14-day combination of lopinavir 400 mg and ritonavir 100 mg every 12 h, ribavirin 400 mg every 12 h, and three doses of 8 million international units of interferon beta-1b on alternate days (combination group) or to 14 days of lopinavir 400 mg and ritonavir 100 mg every 12 h (control group). The primary endpoint was the time to providing a nasopharyngeal swab negative for severe acute respiratory syndrome coronavirus 2 RT-PCR, and was done in the intention-to-treat population. The study is registered with ClinicalTrials.gov, NCT04276688.
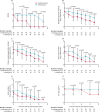
Findings: Between Feb 10 and March 20, 2020, 127 patients were recruited; 86 were randomly assigned to the combination group and 41 were assigned to the control group. The median number of days from symptom onset to start of study treatment was 5 days (IQR 3-7). The combination group had a significantly shorter median time from start of study treatment to negative nasopharyngeal swab (7 days [IQR 5-11]) than the control group (12 days [8-15]; hazard ratio 4·37 [95% CI 1·86-10·24], p=0·0010). Adverse events included self-limited nausea and diarrhoea with no difference between the two groups. One patient in the control group discontinued lopinavir-ritonavir because of biochemical hepatitis. No patients died during the study.
Interpretation: Early triple antiviral therapy was safe and superior to lopinavir-ritonavir alone in alleviating symptoms and shortening the duration of viral shedding and hospital stay in patients with mild to moderate COVID-19. Future clinical study of a double antiviral therapy with interferon beta-1b as a backbone is warranted.
Funding: The Shaw-Foundation, Richard and Carol Yu, May Tam Mak Mei Yin, and Sanming Project of Medicine.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Interferon beta-1b for COVID-19.Lancet. 2020 May 30;395(10238):1670-1671. doi: 10.1016/S0140-6736(20)31101-6. Epub 2020 May 10. Lancet. 2020. PMID: 32401712 Free PMC article. No abstract available.
-
Early triple antiviral therapy for COVID-19.Lancet. 2020 Nov 7;396(10261):1487-1488. doi: 10.1016/S0140-6736(20)32274-1. Lancet. 2020. PMID: 33160566 Free PMC article. No abstract available.
-
Early triple antiviral therapy for COVID-19 - Authors' reply.Lancet. 2020 Nov 7;396(10261):1488. doi: 10.1016/S0140-6736(20)32268-6. Lancet. 2020. PMID: 33160568 Free PMC article. No abstract available.
Similar articles
-
Effectiveness of Interferon Beta 1a, compared to Interferon Beta 1b and the usual therapeutic regimen to treat adults with moderate to severe COVID-19: structured summary of a study protocol for a randomized controlled trial.Trials. 2020 Jun 3;21(1):473. doi: 10.1186/s13063-020-04382-3. Trials. 2020. PMID: 32493468 Free PMC article.
-
Safety and efficacy of antiviral combination therapy in symptomatic patients of Covid-19 infection - a randomised controlled trial (SEV-COVID Trial): A structured summary of a study protocol for a randomized controlled trial.Trials. 2020 Oct 20;21(1):866. doi: 10.1186/s13063-020-04774-5. Trials. 2020. PMID: 33081849 Free PMC article. Clinical Trial.
-
An investigation into the beneficial effects of high-dose interferon beta 1-a, compared to low-dose interferon beta 1-a (the base therapeutic regimen) in moderate to severe COVID-19: A structured summary of a study protocol for a randomized controlled l trial.Trials. 2020 Oct 26;21(1):880. doi: 10.1186/s13063-020-04812-2. Trials. 2020. PMID: 33106183 Free PMC article. Clinical Trial.
-
Compounds with Therapeutic Potential against Novel Respiratory 2019 Coronavirus.Antimicrob Agents Chemother. 2020 Apr 21;64(5):e00399-20. doi: 10.1128/AAC.00399-20. Print 2020 Apr 21. Antimicrob Agents Chemother. 2020. PMID: 32152082 Free PMC article. Review.
-
Clinical Trials of Repurposed Antivirals for SARS-CoV-2.Antimicrob Agents Chemother. 2020 Aug 20;64(9):e01101-20. doi: 10.1128/AAC.01101-20. Print 2020 Aug 20. Antimicrob Agents Chemother. 2020. PMID: 32631826 Free PMC article. Review.
Cited by
-
No Statistically Apparent Difference in Antiviral Effectiveness Observed Among Ribavirin Plus Interferon-Alpha, Lopinavir/Ritonavir Plus Interferon-Alpha, and Ribavirin Plus Lopinavir/Ritonavir Plus Interferon-Alpha in Patients With Mild to Moderate Coronavirus Disease 2019: Results of a Randomized, Open-Labeled Prospective Study.Front Pharmacol. 2020 Jul 14;11:1071. doi: 10.3389/fphar.2020.01071. eCollection 2020. Front Pharmacol. 2020. PMID: 32765274 Free PMC article.
-
Effectiveness of Drug Repurposing and Natural Products Against SARS-CoV-2: A Comprehensive Review.Clin Pharmacol. 2024 Jan 4;16:1-25. doi: 10.2147/CPAA.S429064. eCollection 2024. Clin Pharmacol. 2024. PMID: 38197085 Free PMC article. Review.
-
Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease.Sci Rep. 2021 May 11;11(1):9927. doi: 10.1038/s41598-021-89444-5. Sci Rep. 2021. PMID: 33976287 Free PMC article. Clinical Trial.
-
COVID-19 Therapeutic Options Under Investigation.Front Pharmacol. 2020 Aug 6;11:1196. doi: 10.3389/fphar.2020.01196. eCollection 2020. Front Pharmacol. 2020. PMID: 32848795 Free PMC article. Review.
-
Macrophage responses associated with COVID-19: A pharmacological perspective.Eur J Pharmacol. 2020 Nov 15;887:173547. doi: 10.1016/j.ejphar.2020.173547. Epub 2020 Sep 11. Eur J Pharmacol. 2020. PMID: 32919938 Free PMC article. Review.
References
-
- WHO Coronavirus disease 2019 (COVID-19) situation report–107. May 6, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
-
- Yao XH, Li TY, He ZC. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. (in Chinese). - PubMed
-
- Chan JF, Zhang AJ, Yuan S. Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in golden syrian hamster model: implications for disease pathogenesis and tansmissibility. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa325. publised online March 26. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

