Electrophilic Vinylation of Thiols under Mild and Transition Metal-Free Conditions
- PMID: 32395880
- PMCID: PMC7497129
- DOI: 10.1002/anie.202002936
Electrophilic Vinylation of Thiols under Mild and Transition Metal-Free Conditions
Abstract
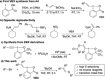
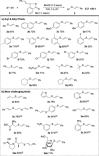
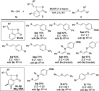
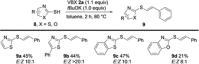
The iodine(III) reagents vinylbenziodoxolones (VBX) were employed to vinylate a series of aliphatic and aromatic thiols, providing E-alkenyl sulfides with complete chemo- and regioselectivity, as well as excellent stereoselectivity. The methodology displays high functional group tolerance and proceeds under mild and transition metal-free conditions without the need for excess substrate or reagents. Mercaptothiazoles could be vinylated under modified conditions, resulting in opposite stereoselectivity compared to previous reactions with vinyliodonium salts. Novel VBX reagents with substituted benziodoxolone cores were prepared, and improved reactivity was discovered with a dimethyl-substituted core.
Keywords: alkenyl sulfides; benziodoxolones; hypervalent compounds; synthetic methods; vinylbenziodoxolones.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Structure-reactivity analysis of novel hypervalent iodine reagents in S-vinylation of thiols.Front Chem. 2024 Feb 29;12:1376948. doi: 10.3389/fchem.2024.1376948. eCollection 2024. Front Chem. 2024. PMID: 38487782 Free PMC article.
-
Synthesis, Characterization and Unusual Reactivity of Vinylbenziodoxolones-Novel Hypervalent Iodine Reagents.Chemistry. 2016 Nov 2;22(45):16066-16070. doi: 10.1002/chem.201603955. Epub 2016 Oct 6. Chemistry. 2016. PMID: 27629653
-
Transition metal-free and regioselective vinylation of phosphine oxides and H-phosphinates with VBX reagents.Chem Commun (Camb). 2020 Nov 19;56(92):14389-14392. doi: 10.1039/d0cc05992g. Chem Commun (Camb). 2020. PMID: 33140771
-
Aryl-, Akynyl-, and Alkenylbenziodoxoles: Synthesis and Synthetic Applications.Molecules. 2023 Feb 24;28(5):2136. doi: 10.3390/molecules28052136. Molecules. 2023. PMID: 36903382 Free PMC article. Review.
-
Atom-economical group-transfer reactions with hypervalent iodine compounds.Beilstein J Org Chem. 2018 May 30;14:1263-1280. doi: 10.3762/bjoc.14.108. eCollection 2018. Beilstein J Org Chem. 2018. PMID: 29977394 Free PMC article. Review.
Cited by
-
Recent Progress in Synthetic Applications of Hypervalent Iodine(III) Reagents.Chem Rev. 2024 Oct 9;124(19):11108-11186. doi: 10.1021/acs.chemrev.4c00303. Epub 2024 Sep 13. Chem Rev. 2024. PMID: 39269928 Free PMC article. Review.
-
Iodine(III)-Mediated Oxidation of Anilines to Construct Dibenzazepines.Chemistry. 2023 Jul 3;29(37):e202301141. doi: 10.1002/chem.202301141. Epub 2023 May 11. Chemistry. 2023. PMID: 37053500 Free PMC article.
-
Transition-Metal-Free Difunctionalization of Sulfur Nucleophiles.Angew Chem Int Ed Engl. 2023 Feb 13;62(8):e202216296. doi: 10.1002/anie.202216296. Epub 2023 Jan 13. Angew Chem Int Ed Engl. 2023. PMID: 36546892 Free PMC article.
-
Structure-reactivity analysis of novel hypervalent iodine reagents in S-vinylation of thiols.Front Chem. 2024 Feb 29;12:1376948. doi: 10.3389/fchem.2024.1376948. eCollection 2024. Front Chem. 2024. PMID: 38487782 Free PMC article.
-
Ritter-type iodo(iii)amidation of unactivated alkynes for the stereoselective synthesis of multisubstituted enamides.Chem Sci. 2021 Nov 4;12(45):15128-15133. doi: 10.1039/d1sc05240c. eCollection 2021 Nov 24. Chem Sci. 2021. PMID: 34909154 Free PMC article.
References
-
- None
-
- Zhdankin V. V., Hypervalent Iodine Chemistry, Wiley, Chichester, 2014;
-
- Wirth T., Topics in Current Chemistry, Vol. 373, Springer International Publishing, Cham, 2016;
-
- Olofsson B., Marek I., Rappoport Z., Patai's Chemistry of Functional Groups, Wiley, Hoboken, 2019, p. 1032.
-
- None

