Pharmacologic TWIK-Related Acid-Sensitive K+ Channel (TASK-1) Potassium Channel Inhibitor A293 Facilitates Acute Cardioversion of Paroxysmal Atrial Fibrillation in a Porcine Large Animal Model
- PMID: 32390491
- PMCID: PMC7660874
- DOI: 10.1161/JAHA.119.015751
Pharmacologic TWIK-Related Acid-Sensitive K+ Channel (TASK-1) Potassium Channel Inhibitor A293 Facilitates Acute Cardioversion of Paroxysmal Atrial Fibrillation in a Porcine Large Animal Model
Abstract
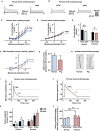
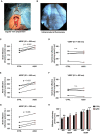
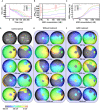
Background The tandem of P domains in a weak inward rectifying K+ channel (TWIK)-related acid-sensitive K+ channel (TASK-1; hK2P3.1) two-pore-domain potassium channel was recently shown to regulate the atrial action potential duration. In the human heart, TASK-1 channels are specifically expressed in the atria. Furthermore, upregulation of atrial TASK-1 currents was described in patients suffering from atrial fibrillation (AF). We therefore hypothesized that TASK-1 channels represent an ideal target for antiarrhythmic therapy of AF. In the present study, we tested the antiarrhythmic effects of the high-affinity TASK-1 inhibitor A293 on cardioversion in a porcine model of paroxysmal AF. Methods and Results Heterologously expressed human and porcine TASK-1 channels are blocked by A293 to a similar extent. Patch clamp measurements from isolated human and porcine atrial cardiomyocytes showed comparable TASK-1 currents. Computational modeling was used to investigate the conditions under which A293 would be antiarrhythmic. German landrace pigs underwent electrophysiological studies under general anesthesia. Paroxysmal AF was induced by right atrial burst stimulation. After induction of AF episodes, intravenous administration of A293 restored sinus rhythm within cardioversion times of 177±63 seconds. Intravenous administration of A293 resulted in significant prolongation of the atrial effective refractory period, measured at cycle lengths of 300, 400 and 500 ms, whereas the surface ECG parameters and the ventricular effective refractory period lengths remained unchanged. Conclusions Pharmacological inhibition of atrial TASK-1 currents exerts antiarrhythmic effects in vivo as well as in silico, resulting in acute cardioversion of paroxysmal AF. Taken together, these experiments indicate the therapeutic potential of A293 for AF treatment.
Keywords: A293; K2P3.1; TASK‐1; antiarrhythmic pharmacotherapy; atrial fibrillation; cardioversion.
Figures



Similar articles
-
Genetic Ablation of TASK-1 (Tandem of P Domains in a Weak Inward Rectifying K+ Channel-Related Acid-Sensitive K+ Channel-1) (K2P3.1) K+ Channels Suppresses Atrial Fibrillation and Prevents Electrical Remodeling.Circ Arrhythm Electrophysiol. 2019 Sep;12(9):e007465. doi: 10.1161/CIRCEP.119.007465. Epub 2019 Sep 13. Circ Arrhythm Electrophysiol. 2019. PMID: 31514528
-
The Experimental TASK-1 Potassium Channel Inhibitor A293 Can Be Employed for Rhythm Control of Persistent Atrial Fibrillation in a Translational Large Animal Model.Front Physiol. 2021 Jan 21;11:629421. doi: 10.3389/fphys.2020.629421. eCollection 2020. Front Physiol. 2021. PMID: 33551849 Free PMC article.
-
Identification of the A293 (AVE1231) Binding Site in the Cardiac Two-Pore-Domain Potassium Channel TASK-1: a Common Low Affinity Antiarrhythmic Drug Binding Site.Cell Physiol Biochem. 2019;52(5):1223-1235. doi: 10.33594/000000083. Cell Physiol Biochem. 2019. PMID: 31001961
-
Inhibition of cardiac two-pore-domain K+ (K2P) channels--an emerging antiarrhythmic concept.Eur J Pharmacol. 2014 Sep 5;738:250-5. doi: 10.1016/j.ejphar.2014.05.056. Epub 2014 Jun 10. Eur J Pharmacol. 2014. PMID: 24927994 Review.
-
Atrial-selective K+ channel blockers: potential antiarrhythmic drugs in atrial fibrillation?Can J Physiol Pharmacol. 2017 Nov;95(11):1313-1318. doi: 10.1139/cjpp-2017-0024. Epub 2017 Jul 24. Can J Physiol Pharmacol. 2017. PMID: 28738160 Review.
Cited by
-
Two-Pore-Domain Potassium (K2P-) Channels: Cardiac Expression Patterns and Disease-Specific Remodelling Processes.Cells. 2021 Oct 27;10(11):2914. doi: 10.3390/cells10112914. Cells. 2021. PMID: 34831137 Free PMC article. Review.
-
Cardiac K+ Channels and Channelopathies.Handb Exp Pharmacol. 2021;267:113-138. doi: 10.1007/164_2021_513. Handb Exp Pharmacol. 2021. PMID: 34247279 Review.
-
Research progress of two-pore potassium channel in myocardial ischemia-reperfusion injury.Front Physiol. 2024 Oct 29;15:1473501. doi: 10.3389/fphys.2024.1473501. eCollection 2024. Front Physiol. 2024. PMID: 39534859 Free PMC article. Review.
-
How synergy between mechanistic and statistical models is impacting research in atrial fibrillation.Front Physiol. 2022 Aug 30;13:957604. doi: 10.3389/fphys.2022.957604. eCollection 2022. Front Physiol. 2022. PMID: 36111152 Free PMC article. Review.
-
The Experimental TASK-1 Potassium Channel Inhibitor A293 Can Be Employed for Rhythm Control of Persistent Atrial Fibrillation in a Translational Large Animal Model.Front Physiol. 2021 Apr 12;12:668267. doi: 10.3389/fphys.2021.668267. eCollection 2021. Front Physiol. 2021. PMID: 33912077 Free PMC article. No abstract available.
References
-
- Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1678. - PubMed
-
- Fabritz L, Guasch E, Antoniades C, Bardinet I, Benninger G, Betts TR, Brand E, Breithardt G, Bucklar‐Suchankova G, Camm AJ, et al. Expert consensus document: defining the major health modifiers causing atrial fibrillation: a roadmap to underpin personalized prevention and treatment. Nat Rev Cardiol. 2016;13:230–237. - PubMed
-
- January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;47:104–132. - PubMed
-
- Goldstein SAN, Bockenhauer D, O'Kelly I, Zilberberg N. Potassium leak channels and the KCNK family two‐P‐domain subunits. Nat Rev Neurosci. 2001;2:175–184. - PubMed
-
- Patel AJ, Honoré E. Molecular physiology of oxygen‐sensitive potassium channels. Eur Respir J. 2001;18:221–227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

