Gene silencing of indoleamine 2,3-dioxygenase 1 inhibits lung cancer growth by suppressing T-cell exhaustion
- PMID: 32382333
- PMCID: PMC7202272
- DOI: 10.3892/ol.2020.11477
Gene silencing of indoleamine 2,3-dioxygenase 1 inhibits lung cancer growth by suppressing T-cell exhaustion
Abstract
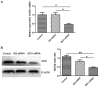
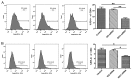
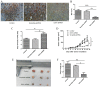
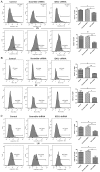
Indoleamine 2,3-dioxygenase 1 (IDO1), which degrades the essential amino acid tryptophan, exerts immunosuppressive functions and serves a crucial role in multiple types tumor progression, including non-small-cell lung cancer (NSCLC) and melanoma. Recent studies have reported that T-cell exhaustion is increased during tumor progression, which impairs the antitumor immune response. However, the association between IDO1 and T-cell exhaustion during tumor progression remains unknown. The present study evaluated the effect of IDO1 on T-cell exhaustion in lung cancer mice. The present study demonstrated that IDO1 knockdown by small interfering RNA in the LLC cell line inhibited T-cell exhaustion. Furthermore, the role of IDO1 in T-cell exhaustion during lung cancer progression was determined in an in vivo mouse model using IDO1 short hairpin RNA (shRNA). The results demonstrated that inhibition of IDO1 activity by shRNA administration in vivo significantly delayed the onset and growth of tumors. In addition, the expression levels of the inhibitory receptors programmed death-1 (PD-1) and B and T lymphocyte attenuator (BTLA) were increased in T-cells from the lung tumor-bearing mice, whereas interleukin-2 (IL-2) and tumor necrosis factor-alpha (TNF-α) levels in serum were decreased compared with the control mice. However, no difference in the absolute number of T cells was observed, including CD4+ and CD8+ T cells. In addition, IDO1 knockdown by shRNA inhibited T-cell exhaustion in lung tumor-bearing mice, which was characterized by decreased expression of PD-1 and BTLA on T cells. By contrast, IL-2 and TNF-α levels in serum were increased in IDO1-shRNA-treated mice. By using a shRNA approach, the present study demonstrated that IDO1 activity may be involved in tumor growth, and that IDO1 silencing may inhibit tumor progression by impeding the process of T-cell exhaustion.
Keywords: T-cell exhaustion; immunotherapy; indoleamine 2,3 dioxygenase 1; lung cancer.
Copyright: © Shang et al.
Figures

Similar articles
-
Gene silencing of indoleamine 2,3-dioxygenase hinders tumor growth through angiogenesis inhibition.Int J Oncol. 2017 Jun;50(6):2136-2144. doi: 10.3892/ijo.2017.3975. Epub 2017 Apr 25. Int J Oncol. 2017. PMID: 28498425
-
Effects of gene silencing of indoleamine 2,3-dioxygenase 1 combined with rosmarinic acid on tumor immune microenvironment in H22 tumor-bearing mice.Int Immunopharmacol. 2023 Jun;119:110193. doi: 10.1016/j.intimp.2023.110193. Epub 2023 Apr 14. Int Immunopharmacol. 2023. PMID: 37062258
-
Upregulation of Indoleamine 2,3-Dioxygenase 1 in Tumor Cells and Tertiary Lymphoid Structures is a Hallmark of Inflamed Non-Small Cell Lung Cancer.Clin Cancer Res. 2023 Dec 1;29(23):4883-4893. doi: 10.1158/1078-0432.CCR-23-1928. Clin Cancer Res. 2023. PMID: 37756581 Free PMC article.
-
The potential of targeting indoleamine 2,3-dioxygenase for cancer treatment.Expert Opin Ther Targets. 2015 May;19(5):605-15. doi: 10.1517/14728222.2014.995092. Epub 2015 Feb 15. Expert Opin Ther Targets. 2015. PMID: 25684107 Review.
-
The interplay between indoleamine 2,3-dioxygenase 1 (IDO1) and cyclooxygenase (COX)-2 in chronic inflammation and cancer.Curr Med Chem. 2011;18(15):2263-71. doi: 10.2174/092986711795656063. Curr Med Chem. 2011. PMID: 21517752 Review.
Cited by
-
SNF5 promotes cell proliferation and immune evasion in non-small cell lung cancer.Bioengineered. 2022 May;13(5):11530-11540. doi: 10.1080/21655979.2022.2068894. Bioengineered. 2022. PMID: 35506290 Free PMC article.
-
Regulation of T cells by myeloid-derived suppressor cells: emerging immunosuppressor in lung cancer.Discov Oncol. 2023 Oct 19;14(1):185. doi: 10.1007/s12672-023-00793-1. Discov Oncol. 2023. PMID: 37857728 Free PMC article. Review.
-
From Melanoma Development to RNA-Modified Dendritic Cell Vaccines: Highlighting the Lessons From the Past.Front Immunol. 2021 Feb 22;12:623639. doi: 10.3389/fimmu.2021.623639. eCollection 2021. Front Immunol. 2021. PMID: 33692796 Free PMC article. Review.
-
IDO1 Modulates the Sensitivity of Epithelial Ovarian Cancer Cells to Cisplatin through ROS/p53-Dependent Apoptosis.Int J Mol Sci. 2022 Oct 9;23(19):12002. doi: 10.3390/ijms231912002. Int J Mol Sci. 2022. PMID: 36233312 Free PMC article.
-
Indoleamine-2,3 dioxygenase: a fate-changer of the tumor microenvironment.Mol Biol Rep. 2023 Jul;50(7):6133-6145. doi: 10.1007/s11033-023-08469-3. Epub 2023 May 22. Mol Biol Rep. 2023. PMID: 37217614 Free PMC article. Review.
References
-
- Speeckaert R, Vermaelen K, van Geel N, Autier P, Lambert J, Haspeslagh M, van Gele M, Thielemans K, Neyns B, Roche N, et al. Indoleamine 2,3-dioxygenase, a new prognostic marker in sentinel lymph nodes of melanoma patients. Eur J Cancer. 2012;48:2004–2011. doi: 10.1016/j.ejca.2011.09.007. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
