Wnt signaling in chondroprogenitors during long bone development and growth
- PMID: 32380258
- PMCID: PMC7354209
- DOI: 10.1016/j.bone.2020.115368
Wnt signaling in chondroprogenitors during long bone development and growth
Abstract
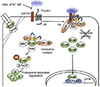
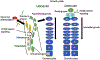
Wnt signaling together with other signaling pathways governs cartilage development and the growth plate function during long bone formation and growth. β-catenin-dependent Wnt signaling is a specific lineage determinant of skeletal mesenchymal cells toward chondrogenic or osteogenic direction. Once cartilage forms and the growth plate organize, Wnt signaling continues to regulate proliferation and differentiation of the growth plate chondrocytes. Although chondrocytes in the growth plate have a high capacity to proliferate, new cells must be supplied to the growth plate from chondroprogenitor population. Advances in in vivo cell tracking techniques have demonstrated the importance of Wnt signaling in driving tissue renewal. The Wnt-responsive cells, genetically marked by the Wnt-reporter system, are found as stem cells in various tissues. Similarly, Wnt-responsive cells are found in the periphery of the growth plate and expanded to constitute entire column structure, indicating that Wnt signaling participates in the regulation of chondroprogenitors in the growth plate. This review will discuss advancements in research of progenitors in the growth plate, specifically focusing on Wnt/β-catenin signaling.
Keywords: Chondroprogenitor; Growth plate; Wnt signaling.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Possible Contribution of Wnt-Responsive Chondroprogenitors to the Postnatal Murine Growth Plate.J Bone Miner Res. 2019 May;34(5):964-974. doi: 10.1002/jbmr.3658. Epub 2019 Jan 28. J Bone Miner Res. 2019. PMID: 30602070 Free PMC article.
-
Thyroid hormone interacts with the Wnt/beta-catenin signaling pathway in the terminal differentiation of growth plate chondrocytes.J Bone Miner Res. 2007 Dec;22(12):1988-95. doi: 10.1359/jbmr.070806. J Bone Miner Res. 2007. PMID: 17708712
-
Roles of Wnt/β-catenin signalling pathway in the bony repair of injured growth plate cartilage in young rats.Bone. 2013 Feb;52(2):651-8. doi: 10.1016/j.bone.2012.10.035. Epub 2012 Nov 10. Bone. 2013. PMID: 23149278
-
The Key Role of Canonical Wnt/β-catenin Signaling in Cartilage Chondrocytes.Curr Drug Targets. 2016;17(4):475-84. doi: 10.2174/1389450116666150825112623. Curr Drug Targets. 2016. PMID: 26302804 Review.
-
Wnt signaling in cartilage development and diseases: lessons from animal studies.Lab Invest. 2016 Feb;96(2):186-96. doi: 10.1038/labinvest.2015.142. Epub 2015 Dec 7. Lab Invest. 2016. PMID: 26641070 Free PMC article. Review.
Cited by
-
Regulation of FGF-2, FGF-18 and Transcription Factor Activity by Perlecan in the Maturational Development of Transitional Rudiment and Growth Plate Cartilages and in the Maintenance of Permanent Cartilage Homeostasis.Int J Mol Sci. 2022 Feb 9;23(4):1934. doi: 10.3390/ijms23041934. Int J Mol Sci. 2022. PMID: 35216048 Free PMC article. Review.
-
The association between DNA methylation and human height and a prospective model of DNA methylation-based height prediction.Hum Genet. 2024 Mar;143(3):401-421. doi: 10.1007/s00439-024-02659-0. Epub 2024 Mar 20. Hum Genet. 2024. PMID: 38507014
-
Transcriptional profiling of human cartilage endplate cells identifies novel genes and cell clusters underlying degenerated and non-degenerated phenotypes.Arthritis Res Ther. 2024 Jan 3;26(1):12. doi: 10.1186/s13075-023-03220-6. Arthritis Res Ther. 2024. PMID: 38173036 Free PMC article.
-
Ocu-miR-10a-5p promotes the chondrogenic differentiation of rabbit BMSCs by targeting BTRC-mediated Wnt/β-catenin signaling pathway.In Vitro Cell Dev Biol Anim. 2024 Apr;60(4):343-353. doi: 10.1007/s11626-024-00888-1. Epub 2024 Mar 19. In Vitro Cell Dev Biol Anim. 2024. PMID: 38504085
-
Overexpression of Fgf18 in cranial neural crest cells recapitulates Pierre Robin sequence in mice.Front Cell Dev Biol. 2024 Apr 17;12:1376814. doi: 10.3389/fcell.2024.1376814. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38694818 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

