Coumarins as Modulators of the Keap1/Nrf2/ARE Signaling Pathway
- PMID: 32377290
- PMCID: PMC7196981
- DOI: 10.1155/2020/1675957
Coumarins as Modulators of the Keap1/Nrf2/ARE Signaling Pathway
Abstract
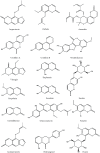
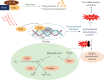
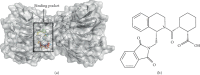
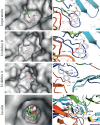
The Keap1/Nrf2/ARE system is a central defensive mechanism against oxidative stress which plays a key role in the pathogenesis and progression of many diseases. Nrf2 is a redox-sensitive transcription factor controlling a variety of downstream antioxidant and cytodefensive genes. Nrf2 has a powerful anti-inflammatory activity mediated via modulating NF-κB. Therefore, pharmacological activation of Nrf2 is a promising therapeutic strategy for the treatment/prevention of several diseases that are underlined by both oxidative stress and inflammation. Coumarins are natural products with promising pharmacological activities, including antioxidant, anticancer, antimicrobial, and anti-inflammatory efficacies. Coumarins are found in many plants, fungi, and bacteria and have been widely used as complementary and alternative medicines. Some coumarins have shown an ability to activate Nrf2 signaling in different cells and animal models. The present review compiles the research findings of seventeen coumarin derivatives of plant origin (imperatorin, visnagin, urolithin B, urolithin A, scopoletin, esculin, esculetin, umbelliferone, fraxetin, fraxin, daphnetin, anomalin, wedelolactone, glycycoumarin, osthole, hydrangenol, and isoimperatorin) as antioxidant and anti-inflammatory agents, emphasizing the role of Nrf2 activation in their pharmacological activities. Additionally, molecular docking simulations were utilized to investigate the potential binding mode of these coumarins with Keap1 as a strategy to disrupt Keap1/Nrf2 protein-protein interaction and activate Nrf2 signaling.
Copyright © 2020 Emad H. M. Hassanein et al.
Conflict of interest statement
The authors report no conflict of interests.
Figures

Similar articles
-
The Keap1/Nrf2-ARE Pathway as a Pharmacological Target for Chalcones.Molecules. 2018 Jul 20;23(7):1803. doi: 10.3390/molecules23071803. Molecules. 2018. PMID: 30037040 Free PMC article. Review.
-
Natural Coumarin Derivatives Activating Nrf2 Signaling Pathway as Lead Compounds for the Design and Synthesis of Intestinal Anti-Inflammatory Drugs.Pharmaceuticals (Basel). 2023 Mar 30;16(4):511. doi: 10.3390/ph16040511. Pharmaceuticals (Basel). 2023. PMID: 37111267 Free PMC article. Review.
-
NF-κB and Keap1 Interaction Represses Nrf2-Mediated Antioxidant Response in Rabbit Hemorrhagic Disease Virus Infection.J Virol. 2020 May 4;94(10):e00016-20. doi: 10.1128/JVI.00016-20. Print 2020 May 4. J Virol. 2020. PMID: 32161178 Free PMC article.
-
The Keap1-Nrf2-ARE Pathway As a Potential Preventive and Therapeutic Target: An Update.Med Res Rev. 2016 Sep;36(5):924-63. doi: 10.1002/med.21396. Epub 2016 May 18. Med Res Rev. 2016. PMID: 27192495 Review.
-
Isoflavone biochanin A, a novel nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response element activator, protects against oxidative damage in HepG2 cells.Biofactors. 2019 Jul;45(4):563-574. doi: 10.1002/biof.1514. Epub 2019 May 27. Biofactors. 2019. PMID: 31131946
Cited by
-
Osthol Ameliorates Kidney Damage and Metabolic Syndrome Induced by a High-Fat/High-Sugar Diet.Int J Mol Sci. 2021 Feb 28;22(5):2431. doi: 10.3390/ijms22052431. Int J Mol Sci. 2021. PMID: 33670975 Free PMC article.
-
The Mechanistic Perspective of Bilobetin Protective Effects against Cisplatin-Induced Testicular Toxicity: Role of Nrf-2/Keap-1 Signaling, Inflammation, and Apoptosis.Biomedicines. 2022 May 13;10(5):1134. doi: 10.3390/biomedicines10051134. Biomedicines. 2022. PMID: 35625871 Free PMC article.
-
Saponins of Tomato Extract Improve Non-Alcoholic Fatty Liver Disease by Regulating Oxidative Stress and Lipid Homeostasis.Antioxidants (Basel). 2023 Oct 12;12(10):1848. doi: 10.3390/antiox12101848. Antioxidants (Basel). 2023. PMID: 37891927 Free PMC article.
-
Pharmacological Effects of Urolithin A and Its Role in Muscle Health and Performance: Current Knowledge and Prospects.Nutrients. 2023 Oct 19;15(20):4441. doi: 10.3390/nu15204441. Nutrients. 2023. PMID: 37892516 Free PMC article. Review.
-
Hsa_circ_0096157 silencing suppresses autophagy and reduces cisplatin resistance in non-small cell lung cancer by weakening the Nrf2/ARE signaling pathway.Mol Biol Rep. 2024 Jun 1;51(1):703. doi: 10.1007/s11033-024-09552-z. Mol Biol Rep. 2024. PMID: 38822881
References
-
- Razavi S. M. Plant coumarins as allelopathic agents. International Journal of Biological Chemistry. 2011;5(1):86–90. doi: 10.3923/ijbc.2011.86.90. - DOI
-
- Iranshahi M., Askari M., Sahebkar A., Adjipavlou-Litina D. Evaluation of antioxidant, anti-inflammatory and lipoxygenase inhibitory activities of the prenylated coumarin umbelliprenin. DARU Journal of Pharmaceutical Sciences. 2009;17(2):99–103.
-
- Gnonlonfin G. J. B., Sanni A., Brimer L. Review Scopoletin – A Coumarin Phytoalexin with Medicinal Properties. Critical Reviews in Plant Sciences. 2012;31(1):47–56. doi: 10.1080/07352689.2011.616039. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

