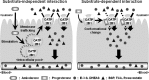
Stimulatory effect on the transport mediated by organic anion transporting polypeptide 2B1
- PMID: 32373198
- PMCID: PMC7193449
- DOI: 10.1016/j.ajps.2019.10.004
Stimulatory effect on the transport mediated by organic anion transporting polypeptide 2B1
Abstract
Drug-drug interaction (DDI) is one of causes of adverse drug events and can result in life-threatening consequences. Organic anion-transporting polypeptide (OATP) 2B1 is a major uptake transporter in the intestine and contributes to transport various clinically used therapeutic agents. The intestine has a high risk of DDI, because it has a special propensity to be exposed to a high concentration of drugs. Thus, understanding drug interaction mediated by OATP2B1 in the absorption process is important for the prevention of adverse drug events, including decrease in the therapeutic effect of co-administered drugs. Acute drug interaction occurs through the direct inhibitory effect on transporters, including OATP2B1. Moreover, some compounds such as clinically used drugs and food components have an acute stimulatory effect on transport of co-administered drugs by OATP2B1. This review summarizes the acute stimulatory effect on the transport mediated by OATP2B1 and discusses the mechanisms of the acute stimulatory effects of compounds. There are two types of acute stimulatory effects, substrate-independent and -dependent interactions on OATP2B1 function. The facilitating translocation of OATP2B1 to the plasma membrane is one of causes for the substrate-independent acute stimulatory effect. On the contrary, the substrate-dependent effect is based on the direct binding to the substrate-binding site or allosteric progesterone-binding site of OATP2B1.
Keywords: Conformational change; Drug interaction; Membrane translocation; OATP2B1; Stimulatory effect.
© 2019 Shenyang Pharmaceutical University. Published by Elsevier B.V.
Figures
Similar articles
-
Quercetin-3-rhamnoglucoside (rutin) stimulates transport of organic anion compounds mediated by organic anion transporting polypeptide 2B1.Biopharm Drug Dispos. 2014 Apr;35(3):173-82. doi: 10.1002/bdd.1882. Epub 2014 Jan 14. Biopharm Drug Dispos. 2014. PMID: 24285294
-
Investigation of Fluorescent Substrates and Substrate-Dependent Interactions of a Drug Transporter Organic Anion Transporting Polypeptide 2B1 (OATP2B1).Pharm Res. 2020 Jun 1;37(6):115. doi: 10.1007/s11095-020-02831-x. Pharm Res. 2020. PMID: 32483763
-
Organic anion-transporting polypeptide (OATP) 2B1 contributes to the cellular uptake of theaflavin.Drug Metab Pharmacokinet. 2017 Apr;32(2):145-150. doi: 10.1016/j.dmpk.2016.11.009. Epub 2016 Nov 22. Drug Metab Pharmacokinet. 2017. PMID: 28190756
-
Organic anion transporting polypeptide 2B1 - More than a glass-full of drug interactions.Pharmacol Ther. 2019 Apr;196:204-215. doi: 10.1016/j.pharmthera.2018.12.009. Epub 2018 Dec 14. Pharmacol Ther. 2019. PMID: 30557631 Review.
-
OATP2B1 - The underrated member of the organic anion transporting polypeptide family of drug transporters?Biochem Pharmacol. 2021 Jun;188:114534. doi: 10.1016/j.bcp.2021.114534. Epub 2021 Mar 29. Biochem Pharmacol. 2021. PMID: 33794186 Review.
Cited by
-
The involvement of human organic anion transporting polypeptides (OATPs) in drug-herb/food interactions.Chin Med. 2020 Jul 9;15:71. doi: 10.1186/s13020-020-00351-9. eCollection 2020. Chin Med. 2020. PMID: 32670395 Free PMC article. Review.
-
Regulation of Drug Transport Proteins-From Mechanisms to Clinical Impact: A White Paper on Behalf of the International Transporter Consortium.Clin Pharmacol Ther. 2022 Sep;112(3):461-484. doi: 10.1002/cpt.2605. Epub 2022 May 24. Clin Pharmacol Ther. 2022. PMID: 35390174 Free PMC article. Review.
-
Organic Anion Transporting Polypeptide 2B1 in Human Fetal Membranes: A Novel Gatekeeper for Drug Transport During Pregnancy?Front Pharmacol. 2021 Dec 20;12:771818. doi: 10.3389/fphar.2021.771818. eCollection 2021. Front Pharmacol. 2021. PMID: 34987396 Free PMC article.
-
Special topic: Emerging role of transporters in drug interaction and delivery.Asian J Pharm Sci. 2020 Mar;15(2):129-130. doi: 10.1016/j.ajps.2020.03.005. Epub 2020 Apr 30. Asian J Pharm Sci. 2020. PMID: 32373194 Free PMC article. No abstract available.
References
-
- Beijer H.J., de Blaey C.J. Hospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studies. Pharm World Sci. 2002;24(2):46–54. - PubMed
-
- Oscanoa T.J., Lizaraso F., Carvajal A. Hospital admissions due to adverse drug reactions in the elderly. A Meta-anal Eur J Clin Pharmacol. 2017;73(6):759–770. - PubMed
-
- Food and Drug Administration; Drug Interactions & Labeling. https://www.fda.gov/drugs/development-resources/drug-interactions-labeling (Accessed 10 July 2019).
-
- Day R.O., Snowden L., McLachlan A.J. Life-threatening drug interactions: what the physician needs to know. Intern Med J. 2017;47(5):501–512. - PubMed
-
- Doan J., Zakrzewski-Jakubiak H., Roy J., Turgeon J., Tannenbaum C. Prevalence and risk of potential cytochrome P450-mediated drug-drug interactions in older hospitalized patients with polypharmacy. Ann Pharmacother. 2013;47(3):324–332. - PubMed
Publication types
LinkOut - more resources
Full Text Sources