Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis
- PMID: 32372026
- PMCID: PMC7199650
- DOI: 10.1038/s41375-020-0848-3
Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis
Abstract
We performed a meta-analysis to determine safety and efficacy of corticosteroids in SARS-CoV-2, SARS-CoV, and MERS-CoV infections. We searched PubMed, Web of Science, Medline, WanFang Chinese database, and ZhiWang Chinese database using Boolean operators and search terms covering SARS-CoV-2, SARS-CoV, OR MERS-CoV AND corticosteroids to find appropriate studies. Review Manager 5.3 was used to analyze results of meta-analysis. Observational studies were analyzed for quality using the modified Newcastle-Ottawa scale and randomized clinical trials, using the Jadad scale. Subjects were divided into those with severe-only and other (severe and not severe) cohorts based on published criteria. Efficacy endpoints studied included mortality, hospitalization duration, rates of intensive care unit (ICU) admission, use of mechanical ventilation, and a composite endpoint (death, ICU admission, or mechanical ventilation). We included 11 reports including 10 cohort studies and 1 randomized clinical trial involving 5249 subjects (2003-2020). Two discussed the association of corticosteroids and virus clearing and 10 explored how corticosteroids impacted mortality, hospitalization duration, use of mechanical ventilation, and a composite endpoint. Corticosteroid use was associated with delayed virus clearing with a mean difference (MD) = 3.78 days (95% confidence Interval [CI] = 1.16, 6.41 days; I2 = 0%). There was no significant reduction in deaths with relative Risk Ratio (RR) = 1.07 (90% CI = 0.81; 1.42; I2 = 80%). Hospitalization duration was prolonged and use of mechanical ventilation increased. In conclusion, corticosteroid use in subjects with SARS-CoV-2, SARS-CoV, and MERS-CoV infections delayed virus clearing and did not convincingly improve survival, reduce hospitalization duration or ICU admission rate and/or use of mechanical ventilation. There were several adverse effects. Because of a preponderance of observational studies in the dataset and selection and publication biases our conclusions, especially regarding SARS-CoV-2, need confirmation in a randomized clinical trial. In the interim we suggest caution using corticosteroids in persons with COVID-19.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
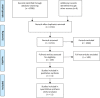
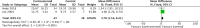

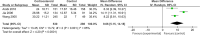
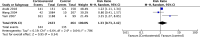
Similar articles
-
SARS, MERS and SARS-CoV-2 (COVID-19) treatment: a patent review.Expert Opin Ther Pat. 2020 Aug;30(8):567-579. doi: 10.1080/13543776.2020.1772231. Epub 2020 Jun 7. Expert Opin Ther Pat. 2020. PMID: 32429703 Review.
-
Remdesivir against COVID-19 and Other Viral Diseases.Clin Microbiol Rev. 2020 Oct 14;34(1):e00162-20. doi: 10.1128/CMR.00162-20. Print 2020 Dec 16. Clin Microbiol Rev. 2020. PMID: 33055231 Free PMC article. Review.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Efficacy and safety of current therapeutic options for COVID-19 - lessons to be learnt from SARS and MERS epidemic: A systematic review and meta-analysis.Pharmacol Res. 2020 Jul;157:104872. doi: 10.1016/j.phrs.2020.104872. Epub 2020 Apr 30. Pharmacol Res. 2020. PMID: 32360583 Free PMC article.
-
Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis.CMAJ. 2020 Jul 6;192(27):E756-E767. doi: 10.1503/cmaj.200645. Epub 2020 May 14. CMAJ. 2020. PMID: 32409522 Free PMC article.
Cited by
-
Severity and Mortality Associated with Steroid Use among Patients with COVID-19: A Systematic Review and Meta-Analysis.Interdiscip Perspect Infect Dis. 2021 May 6;2021:6650469. doi: 10.1155/2021/6650469. eCollection 2021. Interdiscip Perspect Infect Dis. 2021. PMID: 34035806 Free PMC article. Review.
-
Perspective: Cell therapy, SARS-CoV-2, COVID-19, and James Lind.Adv Cell Gene Ther. 2020 Oct;3(4):e88. doi: 10.1002/acg2.88. Epub 2020 May 21. Adv Cell Gene Ther. 2020. PMID: 32838211 Free PMC article. No abstract available.
-
[Emergency and intensive care medicine aspects of COVID-19 infections].Radiologe. 2020 Oct;60(10):899-907. doi: 10.1007/s00117-020-00742-x. Radiologe. 2020. PMID: 32840663 Free PMC article. Review. German.
-
A Comprehensive Review of Tocilizumab in COVID-19 Acute Respiratory Distress Syndrome.J Clin Pharmacol. 2020 Sep;60(9):1131-1146. doi: 10.1002/jcph.1693. Epub 2020 Jul 27. J Clin Pharmacol. 2020. PMID: 32557541 Free PMC article. Review.
-
Gastroenteritis and cardiogenic shock in a healthcare worker: a case report of COVID-19 myocarditis confirmed with serology.Eur Heart J Case Rep. 2021 Feb 8;5(2):ytab013. doi: 10.1093/ehjcr/ytab013. eCollection 2021 Feb. Eur Heart J Case Rep. 2021. PMID: 34104859 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- 81873428/National Natural Science Foundation of China (National Science Foundation of China)/International
- 81660682/National Natural Science Foundation of China (National Science Foundation of China)/International
- 2017ZT07S096/Guangdong Innovative and Entrepreneurial Research Team Program/International
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

