Serum alpha-mannosidase as an additional barrier to eliciting oligomannose-specific HIV-1-neutralizing antibodies
- PMID: 32371950
- PMCID: PMC7200719
- DOI: 10.1038/s41598-020-64500-8
Serum alpha-mannosidase as an additional barrier to eliciting oligomannose-specific HIV-1-neutralizing antibodies
Abstract
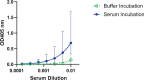
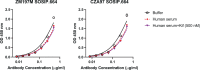
Oligomannose-type glycans on HIV-1 gp120 form a patch that is targeted by several broadly neutralizing antibodies (bnAbs) and that therefore is of interest to vaccine design. However, attempts to elicit similar oligomannose-specific bnAbs by immunizing with oligomannosidic glycoconjugates have only been modestly successful so far. A common assumption is that eliciting oligomannose-specific bnAbs is hindered by B cell tolerance, resulting from the presented oligomannosides being sensed as self molecules. Here, we present data, along with existing scientific evidence, supporting an additional, or perhaps alternate, explanation: serum mannosidase trimming of the presented oligomannosides in vivo. Mannosidase trimming lessens the likelihood of eliciting antibodies with capacity to bind full-sized oligomannose, which typifies the binding mode of existing bnAbs to the oligomannose patch. The rapidity of the observed trimming suggests the need for immunization strategies and/or synthetic glycosides that readily avoid or resist mannosidase trimming upon immunization and can overcome possible tolerance restrictions.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Bacterially derived synthetic mimetics of mammalian oligomannose prime antibody responses that neutralize HIV infectivity.Nat Commun. 2017 Nov 17;8(1):1601. doi: 10.1038/s41467-017-01640-y. Nat Commun. 2017. PMID: 29150603 Free PMC article.
-
Vaccination with Glycan-Modified HIV NFL Envelope Trimer-Liposomes Elicits Broadly Neutralizing Antibodies to Multiple Sites of Vulnerability.Immunity. 2019 Nov 19;51(5):915-929.e7. doi: 10.1016/j.immuni.2019.10.008. Epub 2019 Nov 12. Immunity. 2019. PMID: 31732167 Free PMC article.
-
Potent Induction of Envelope-Specific Antibody Responses by Virus-Like Particle Immunogens Based on HIV-1 Envelopes from Patients with Early Broadly Neutralizing Responses.J Virol. 2022 Jan 12;96(1):e0134321. doi: 10.1128/JVI.01343-21. Epub 2021 Oct 20. J Virol. 2022. PMID: 34668778 Free PMC article.
-
Development of an anti-HIV vaccine eliciting broadly neutralizing antibodies.AIDS Res Ther. 2017 Sep 12;14(1):50. doi: 10.1186/s12981-017-0178-3. AIDS Res Ther. 2017. PMID: 28893278 Free PMC article. Review.
-
Development of broadly neutralizing antibodies in HIV-1 infected elite neutralizers.Retrovirology. 2018 Sep 5;15(1):61. doi: 10.1186/s12977-018-0443-0. Retrovirology. 2018. PMID: 30185183 Free PMC article. Review.
Cited by
-
Synthesis of Mannosidase-Stable Man3 and Man4 Glycans Containing S-linked Manα1→2Man Termini.Org Lett. 2021 Apr 16;23(8):3053-3057. doi: 10.1021/acs.orglett.1c00726. Epub 2021 Apr 1. Org Lett. 2021. PMID: 33793242 Free PMC article.
-
Synthetic Neoglycoconjugates of Hepta- and Nonamannoside Ligands for Eliciting Oligomannose-Specific HIV-1-Neutralizing Antibodies.Chembiochem. 2022 Apr 5;23(7):e202200061. doi: 10.1002/cbic.202200061. Epub 2022 Feb 11. Chembiochem. 2022. PMID: 35104013 Free PMC article.
-
Immunogenicity Evaluation of N-Glycans Recognized by HIV Broadly Neutralizing Antibodies.ACS Chem Biol. 2021 Oct 15;16(10):2016-2025. doi: 10.1021/acschembio.1c00375. Epub 2021 Aug 15. ACS Chem Biol. 2021. PMID: 34649433 Free PMC article.
-
A glycoside analog of mammalian oligomannose formulated with a TLR4-stimulating adjuvant elicits HIV-1 cross-reactive antibodies.Sci Rep. 2021 Feb 25;11(1):4637. doi: 10.1038/s41598-021-84116-w. Sci Rep. 2021. PMID: 33633304 Free PMC article.
-
Fluorine-Directed Automated Mannoside Assembly.Angew Chem Int Ed Engl. 2023 Jan 16;62(3):e202213304. doi: 10.1002/anie.202213304. Epub 2022 Dec 12. Angew Chem Int Ed Engl. 2023. PMID: 36331042 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

