Effect of AmyTrap, an amyloid-β binding drug, on Aβ induced mitochondrial dysfunction and tau phosphorylation in cultured neuroblastoma cells
- PMID: 32367269
- PMCID: PMC7358124
- DOI: 10.1007/s11011-019-00520-2
Effect of AmyTrap, an amyloid-β binding drug, on Aβ induced mitochondrial dysfunction and tau phosphorylation in cultured neuroblastoma cells
Abstract
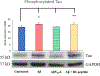
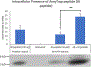
Alzheimer's Disease (AD) is the most common cause of dementia, affecting 25 million people worldwide. Accumulation of Amyloid-β (Aβ) in the mitochondria has been shown to adversely affect key enzymes including pyruvate dehydrogenase (PDH), succinate dehydrogenase (SDH), oxoglutarate dehydrogenase (OGDH). Accumulation of Aβ is also believed to increase Tau expression and pathology. Tau, in its toxic state, results in synaptic damage causing memory and cognitive dysfunction. We are developing a drug to treat AD namely AmyTrap. The active pharmacological ingredient is a retro inverso, Aβ-binding peptide which sequesters Aβ. We wanted to examine the effect of AmyTrap peptide on Aβ-induced mitochondrial dysfunction and Tau phosphorylation. Therefore, we treated SH-SY5Y neuroblastoma cells with wild-type Aβ, a mutant AβY10A, AmyTrap peptide (RI-peptide), or Aβ and RI-peptide for 72 h. The mutant AβY10A is known to impact the self-aggregating property of Aβ as this Tyr10 is essential for self-aggregation. As expected, AβY10A reversed PDH, OGDH and SDH dysfunction to near normal levels. Further, AβY10A successfully reversed Tau phosphorylation, suggesting that Tyr10 is also associated with Aβ-induced cytotoxicity. RI-peptide was able to significantly reverse SDH dysfunction with limited effect on PDH and Tau phosphorylation. The findings are suggestive that the Tyr10 on Aβ plays a critical role in the self-aggregation. Further studies are warranted to expand these findings.
Keywords: Alzheimer’s disease; AmyTrap; Amyloid; Mitochondria; Retro-inverso peptide; SH-SY5Y neuroblastoma cells.
Conflict of interest statement
Declaration of Interest: None
Figures


Similar articles
-
Activated human microglia stimulate neuroblastoma cells to upregulate production of beta amyloid protein and tau: implications for Alzheimer's disease pathogenesis.Neurobiol Aging. 2015 Jan;36(1):42-52. doi: 10.1016/j.neurobiolaging.2014.07.024. Epub 2014 Jul 24. Neurobiol Aging. 2015. PMID: 25169677
-
BRI2 ectodomain affects Aβ42 fibrillation and tau truncation in human neuroblastoma cells.Cell Mol Life Sci. 2015 Apr;72(8):1599-611. doi: 10.1007/s00018-014-1769-y. Epub 2014 Oct 22. Cell Mol Life Sci. 2015. PMID: 25336154 Free PMC article.
-
Synaptic Mitochondria: An Early Target of Amyloid-β and Tau in Alzheimer's Disease.J Alzheimers Dis. 2021;84(4):1391-1414. doi: 10.3233/JAD-215139. J Alzheimers Dis. 2021. PMID: 34719499 Review.
-
Antibodies against beta-amyloid reduce Abeta oligomers, glycogen synthase kinase-3beta activation and tau phosphorylation in vivo and in vitro.J Neurosci Res. 2006 Feb 15;83(3):374-84. doi: 10.1002/jnr.20734. J Neurosci Res. 2006. PMID: 16385556
-
Amyloid Beta and Phosphorylated Tau-Induced Defective Autophagy and Mitophagy in Alzheimer's Disease.Cells. 2019 May 22;8(5):488. doi: 10.3390/cells8050488. Cells. 2019. PMID: 31121890 Free PMC article. Review.
Cited by
-
Metabolic and Cellular Compartments of Acetyl-CoA in the Healthy and Diseased Brain.Int J Mol Sci. 2022 Sep 3;23(17):10073. doi: 10.3390/ijms231710073. Int J Mol Sci. 2022. PMID: 36077475 Free PMC article. Review.
-
Opposite Roles of Co-enzyme Q10 and Formaldehyde in Neurodegenerative Diseases.Am J Alzheimers Dis Other Demen. 2022 Jan-Dec;37:15333175221143274. doi: 10.1177/15333175221143274. Am J Alzheimers Dis Other Demen. 2022. PMID: 36455136 Free PMC article. Review.
-
Identification of core genes in prefrontal cortex and hippocampus of Alzheimer's disease based on mRNA-miRNA network.J Cell Mol Med. 2022 Dec;26(23):5779-5793. doi: 10.1111/jcmm.17593. Epub 2022 Nov 19. J Cell Mol Med. 2022. PMID: 36401602 Free PMC article.
References
-
- Agholme L, Lindström T, Kågedal K, Marcusson J, Hallbeck M. An in vitro model for neuroscience: differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J Alzheimers Dis. 2010. January 1;20(4):1069–1082. - PubMed
-
- Alzheimer’s Association, 2006. Early onset dementia: a national challenge, a future crisis Alzheimer’s Association, Washington, DC.
-
- Alzheimer’s Association, 2012. Alzheimer’s Disease Facts and Figures, Available at: http://www.alz.org/downloads/Facts_Figures. - PubMed
-
- Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann Neurol. 2005. May;57(5):695–703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

