Multifaceted Functions of Host Cell Caveolae/Caveolin-1 in Virus Infections
- PMID: 32357558
- PMCID: PMC7291293
- DOI: 10.3390/v12050487
Multifaceted Functions of Host Cell Caveolae/Caveolin-1 in Virus Infections
Abstract
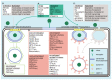
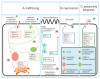
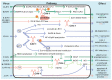
Virus infection has drawn extensive attention since it causes serious or even deadly diseases, consequently inducing a series of social and public health problems. Caveolin-1 is the most important structural protein of caveolae, a membrane invagination widely known for its role in endocytosis and subsequent cytoplasmic transportation. Caveolae/caveolin-1 is tightly associated with a wide range of biological processes, including cholesterol homeostasis, cell mechano-sensing, tumorigenesis, and signal transduction. Intriguingly, the versatile roles of caveolae/caveolin-1 in virus infections have increasingly been appreciated. Over the past few decades, more and more viruses have been identified to invade host cells via caveolae-mediated endocytosis, although other known pathways have been explored. The subsequent post-entry events, including trafficking, replication, assembly, and egress of a large number of viruses, are caveolae/caveolin-1-dependent. Deprivation of caveolae/caveolin-1 by drug application or gene editing leads to abnormalities in viral uptake, viral protein expression, or virion release, whereas the underlying mechanisms remain elusive and must be explored holistically to provide potential novel antiviral targets and strategies. This review recapitulates our current knowledge on how caveolae/caveolin-1 functions in every step of the viral infection cycle and various relevant signaling pathways, hoping to provide a new perspective for future viral cell biology research.
Keywords: caveolae; caveolin-1; signaling pathway; virus assembly; virus egress; virus entry; virus life cycle; virus replication; virus trafficking.
Conflict of interest statement
All the authors declare no conflict of interest.
Figures
Similar articles
-
Recent progress in the topology, structure, and oligomerization of caveolin: a building block of caveolae.Curr Top Membr. 2015;75:305-36. doi: 10.1016/bs.ctm.2015.03.007. Epub 2015 Apr 11. Curr Top Membr. 2015. PMID: 26015287 Review.
-
Clathrin- and caveolin-independent entry of human papillomavirus type 16--involvement of tetraspanin-enriched microdomains (TEMs).PLoS One. 2008 Oct 2;3(10):e3313. doi: 10.1371/journal.pone.0003313. PLoS One. 2008. PMID: 18836553 Free PMC article.
-
Membrane microdomains, caveolae, and caveolar endocytosis of sphingolipids.Mol Membr Biol. 2006 Jan-Feb;23(1):101-10. doi: 10.1080/09687860500460041. Mol Membr Biol. 2006. PMID: 16611585 Review.
-
Caveolin-1: A Promising Therapeutic Target for Diverse Diseases.Curr Mol Pharmacol. 2022;15(5):701-715. doi: 10.2174/1874467214666211130155902. Curr Mol Pharmacol. 2022. PMID: 34847854 Review.
-
Oxidative stress induces caveolin 1 degradation and impairs caveolae functions in skeletal muscle cells.PLoS One. 2015 Mar 23;10(3):e0122654. doi: 10.1371/journal.pone.0122654. eCollection 2015. PLoS One. 2015. PMID: 25799323 Free PMC article.
Cited by
-
Immunoexpression Patterns of Megalin, Cubilin, Caveolin-1, Gipc1 and Dab2IP in the Embryonic and Postnatal Development of the Kidneys in Yotari (Dab1-/-) Mice.Biomedicines. 2024 Jul 11;12(7):1542. doi: 10.3390/biomedicines12071542. Biomedicines. 2024. PMID: 39062115 Free PMC article.
-
Membrane Rafts: Portals for Viral Entry.Front Microbiol. 2021 Feb 4;12:631274. doi: 10.3389/fmicb.2021.631274. eCollection 2021. Front Microbiol. 2021. PMID: 33613502 Free PMC article. Review.
-
Imaging Endocytosis Dynamics in Health and Disease.Membranes (Basel). 2022 Apr 1;12(4):393. doi: 10.3390/membranes12040393. Membranes (Basel). 2022. PMID: 35448364 Free PMC article. Review.
-
D155Y substitution of SARS-CoV-2 ORF3a weakens binding with Caveolin-1.Comput Struct Biotechnol J. 2022;20:766-778. doi: 10.1016/j.csbj.2022.01.017. Epub 2022 Jan 31. Comput Struct Biotechnol J. 2022. PMID: 35126886 Free PMC article.
-
Roles of antiviral sensing and type I interferon signaling in the restriction of SARS-CoV-2 replication.iScience. 2022 Jan 21;25(1):103553. doi: 10.1016/j.isci.2021.103553. Epub 2021 Dec 3. iScience. 2022. PMID: 34877479 Free PMC article.
References
-
- China.org.cn Public Health Emergency of International Concern (The Fight Against COVID-19) [(accessed on 6 March 2020)]; Available online: http://www.china.org.cn/english/china_key_words/2020-03/06/content_75783....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

