Data-Driven Modeling Identifies TIRAP-Independent MyD88 Activation Complex and Myddosome Assembly Strategy in LPS/TLR4 Signaling
- PMID: 32357531
- PMCID: PMC7246728
- DOI: 10.3390/ijms21093061
Data-Driven Modeling Identifies TIRAP-Independent MyD88 Activation Complex and Myddosome Assembly Strategy in LPS/TLR4 Signaling
Abstract
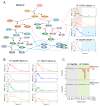
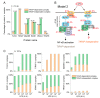
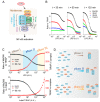
TLR4 complexes are essential for the initiation of the LPS-induced innate immune response. The Myddosome, which mainly contains TLR4, TIRAP, MyD88, IRAK1/4 and TRAF6 proteins, is regarded as a major complex of TLR4. Although the Myddosome has been well studied, a quantitative description of the Myddosome assembly dynamics is still lacking. Furthermore, whether some unknown TLR4 complexes exist remains unclear. In this study, we constructed a SWATH-MS data-based mathematical model that describes the component assembly dynamics of TLR4 complexes. In addition to Myddosome, we suggest that a TIRAP-independent MyD88 activation complex is formed upon LPS stimulation, in which TRAF6 is not included. Furthermore, quantitative analysis reveals that the distribution of components in TIRAP-dependent and -independent MyD88 activation complexes are LPS stimulation-dependent. The two complexes compete for recruiting IRAK1/4 proteins. MyD88 forms higher-order assembly in the Myddosome and we show that the strategy to form higher-order assembly is also LPS stimulation-dependent. MyD88 forms a long chain upon weak stimulation, but forms a short chain upon strong stimulation. Higher-order assembly of MyD88 is directly determined by the level of TIRAP in the Myddosome, providing a formation mechanism for efficient signaling transduction. Taken together, our study provides an enhanced understanding of component assembly dynamics and strategies in TLR4 complexes.
Keywords: LPS signaling; SWATH-MS; TLR4 complexes; higher-order assembly strategy; mathematical modeling; systems biology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Src family kinase tyrosine phosphorylates Toll-like receptor 4 to dissociate MyD88 and Mal/Tirap, suppressing LPS-induced inflammatory responses.Biochem Pharmacol. 2018 Jan;147:119-127. doi: 10.1016/j.bcp.2017.11.015. Epub 2017 Nov 23. Biochem Pharmacol. 2018. PMID: 29175418 Free PMC article.
-
Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4.Nature. 2002 Nov 21;420(6913):324-9. doi: 10.1038/nature01182. Nature. 2002. PMID: 12447441
-
TRAF6 protein couples Toll-like receptor 4 signaling to Src family kinase activation and opening of paracellular pathway in human lung microvascular endothelia.J Biol Chem. 2012 May 11;287(20):16132-45. doi: 10.1074/jbc.M111.310102. Epub 2012 Mar 23. J Biol Chem. 2012. PMID: 22447928 Free PMC article.
-
Modulation of Toll-interleukin 1 receptor mediated signaling.J Mol Med (Berl). 2005 Apr;83(4):258-66. doi: 10.1007/s00109-004-0622-4. Epub 2005 Jan 21. J Mol Med (Berl). 2005. PMID: 15662540 Review.
-
Understanding early TLR signaling through the Myddosome.J Leukoc Biol. 2019 Feb;105(2):339-351. doi: 10.1002/JLB.MR0318-096R. Epub 2018 Sep 26. J Leukoc Biol. 2019. PMID: 30256449 Review.
Cited by
-
TIRAP in the Mechanism of Inflammation.Front Immunol. 2021 Jul 8;12:697588. doi: 10.3389/fimmu.2021.697588. eCollection 2021. Front Immunol. 2021. PMID: 34305934 Free PMC article. Review.
-
scGIR: deciphering cellular heterogeneity via gene ranking in single-cell weighted gene correlation networks.Brief Bioinform. 2024 Jan 22;25(2):bbae091. doi: 10.1093/bib/bbae091. Brief Bioinform. 2024. PMID: 38487851 Free PMC article.
-
Comparative characterization of microRNA-71 of Echinococcus granulosus exosomes.Parasite. 2023;30:55. doi: 10.1051/parasite/2023060. Epub 2023 Dec 11. Parasite. 2023. PMID: 38084936 Free PMC article.
-
Dear-DIAXMBD: Deep Autoencoder Enables Deconvolution of Data-Independent Acquisition Proteomics.Research (Wash D C). 2023 Jun 26;6:0179. doi: 10.34133/research.0179. eCollection 2023. Research (Wash D C). 2023. PMID: 37377457 Free PMC article.
-
The role and mechanisms of gram-negative bacterial outer membrane vesicles in inflammatory diseases.Front Immunol. 2023 Jun 16;14:1157813. doi: 10.3389/fimmu.2023.1157813. eCollection 2023. Front Immunol. 2023. PMID: 37398647 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

