Estrogen receptor α promotes lung cancer cell invasion via increase of and cross-talk with infiltrated macrophages through the CCL2/CCR2/MMP9 and CXCL12/CXCR4 signaling pathways
- PMID: 32356397
- PMCID: PMC7400793
- DOI: 10.1002/1878-0261.12701
Estrogen receptor α promotes lung cancer cell invasion via increase of and cross-talk with infiltrated macrophages through the CCL2/CCR2/MMP9 and CXCL12/CXCR4 signaling pathways
Abstract
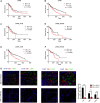
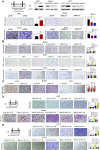
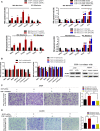
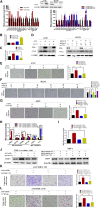
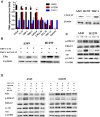
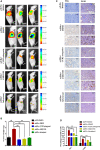
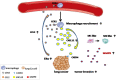
Data analysis of clinical samples suggests that higher estrogen receptor α (ERα) expression could be associated with worse overall survival in some patients with non-small-cell lung cancer (NSCLC). Immunofluorescence results further showed that higher ERα expression was linked to larger numbers of infiltrated macrophages in NSCLC tissues. However, the detailed mechanisms underlying this phenomenon remain unclear. Results from in vitro studies with multiple cell lines revealed that, in NSCLC cells, ERα can activate the CCL2/CCR2 axis to promote macrophage infiltration, M2 polarization, and MMP9 production, which can then increase NSCLC cell invasion. Mechanistic studies using chromatin immunoprecipitation and promoter luciferase assays demonstrated that ERα could bind to estrogen response elements (EREs) on the CCL2 promoter to increase CCL2 expression. Furthermore, ERα-increased macrophage infiltration can induce a positive feedback mechanism to increase lung cancer cell ERα expression via the up-regulation of the CXCL12/CXCR4 pathway. Targeting these newly identified pathways, NSCLC ERα-increased macrophage infiltration or the macrophage-to-NSCLC CXCL12/CXCR4/ERα signal, with anti-estrogens or CCR2/CXCR4 antagonists, may help in the development of new alternative therapies to better treat NSCLC.
Keywords: estrogen receptor α; macrophage; non-small-cell lung cancer.
© 2020 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Hypoxia-inducible factor 1α (HIF-1α) and reactive oxygen species (ROS) mediates radiation-induced invasiveness through the SDF-1α/CXCR4 pathway in non-small cell lung carcinoma cells.Oncotarget. 2015 May 10;6(13):10893-907. doi: 10.18632/oncotarget.3535. Oncotarget. 2015. PMID: 25843954 Free PMC article.
-
Macrophage and cancer cell cross-talk via CCR2 and CX3CR1 is a fundamental mechanism driving lung cancer.Am J Respir Crit Care Med. 2015 Feb 15;191(4):437-47. doi: 10.1164/rccm.201406-1137OC. Am J Respir Crit Care Med. 2015. PMID: 25536148
-
Inflammatory CXCL12-CXCR4/CXCR7 axis mediates G-protein signaling pathway to influence the invasion and migration of nasopharyngeal carcinoma cells.Tumour Biol. 2016 Jun;37(6):8169-79. doi: 10.1007/s13277-015-4686-2. Epub 2015 Dec 29. Tumour Biol. 2016. PMID: 26715277
-
CXCR4/CXCL12 in non-small-cell lung cancer metastasis to the brain.Int J Mol Sci. 2013 Jan 15;14(1):1713-27. doi: 10.3390/ijms14011713. Int J Mol Sci. 2013. PMID: 23322021 Free PMC article. Review.
-
A meta-analysis for CXCR4 as a prognostic marker and potential drug target in non-small cell lung cancer.Drug Des Devel Ther. 2015 Jun 24;9:3267-78. doi: 10.2147/DDDT.S81564. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26150700 Free PMC article. Review.
Cited by
-
C-C motif chemokine ligand 2/C-C receptor 2 is associated with glioma recurrence and poor survival.Exp Ther Med. 2021 Jun;21(6):564. doi: 10.3892/etm.2021.9996. Epub 2021 Mar 26. Exp Ther Med. 2021. PMID: 33850536 Free PMC article.
-
The association between different hormone replacement therapy use and the incidence of lung cancer: a systematic review and meta-analysis.J Thorac Dis. 2022 Feb;14(2):381-395. doi: 10.21037/jtd-22-48. J Thorac Dis. 2022. PMID: 35280481 Free PMC article.
-
Construction and Validation of a Novel Immune-Related Gene Pairs-Based Prognostic Model in Lung Adenocarcinoma.Cancer Control. 2023 Jan-Dec;30:10732748221150227. doi: 10.1177/10732748221150227. Cancer Control. 2023. PMID: 36625357 Free PMC article.
-
Pan-Cancer Prognostic Role and Targeting Potential of the Estrogen-Progesterone Axis.Front Oncol. 2021 Jul 12;11:636365. doi: 10.3389/fonc.2021.636365. eCollection 2021. Front Oncol. 2021. PMID: 34322374 Free PMC article.
-
The genomic landscape of the immune system in lung cancer: present insights and continuing investigations.Front Genet. 2024 Jun 25;15:1414487. doi: 10.3389/fgene.2024.1414487. eCollection 2024. Front Genet. 2024. PMID: 38983267 Free PMC article. Review.
References
-
- Albain KS, Crowley JJ, LeBlanc M and Livingston RB (1991) Survival determinants in extensive‐stage non‐small‐cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol 9, 1618–1626. - PubMed
-
- Ao J‐Y, Zhu X‐D, Chai Z‐T, Cai H, Zhang Y‐Y, Zhang K‐Z, Kong L‐Q, Zhang N, Ye B‐G, Ma D‐N et al (2017) Colony‐stimulating factor 1 receptor blockade inhibits tumor growth by altering the polarization of tumor‐associated Macrophages in Hepatocellular Carcinoma. Mol Cancer Ther 16, 1544–1554. - PubMed
-
- Bröker LE, Huisman C, Span SW, Rodriguez JA, Kruyt FAE and Giaccone G (2004) Cathepsin B Mediates caspase‐independent cell death induced by microtubule stabilizing agents in non‐small cell lung cancer cells. Can Res 64, 27–30. - PubMed
-
- Brueckl WM, Al‐Batran SE, Ficker JH, Claas S, Atmaca A, Hartmann A, Rieker RJ and Wirtz RM (2013) Prognostic and predictive value of estrogen receptor 1 expression in completely resected non‐small cell lung cancer. Int J Cancer 133, 1825–1831. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

